The Dangers and the Diagnosis of CLMK
Despite many advances, the threat of contact lens-related microbial keratitis (CLMK) has not retreated.
By Jaya Sowjanya Siddireddy, PhD
 |
Release Date: August 15, 2019
Expiration Date: August 15, 2022
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss the incidence and types of MK, including bacterial, fungal and Acanthamoeba infections.
- Identify the many modifiable and non-modifiable risk factors associated with CLMK.
- Provide prompt and correct diagnosis of MK, followed by effective pharmaceutical therapy or corneal procedure.
- Educate patients about proper contact lens wear, lens replacement, and cleaning and disinfecting lenses and cases.
Target Audience: This activity is intended for optometrists engaged in the care of patients with contact lens-related microbial keratitis.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Jaya Sowjanya Siddireddy, PhD, School of Optometry and Vision Science, University of New South Wales.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 63763-CL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Siddireddy has nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
Microbial keratitis (MK) is a rare and acute corneal disease that can lead to severe visual disability.1 The severity of the infection depends on the underlying condition of the cornea and the pathogenicity of the microbe. Although it used to occur mostly with predisposing factors such as ocular trauma and ocular surface diseases, an increase in contact lens wear over the last decade has caused a dramatic rise in the prevalence of contact lens-related microbial keratitis (CLMK).2-7 Prognosis of this infectious disease is usually poor if aggressive and appropriate therapy is not initiated promptly.2,8,9
Contact lens wear has been identified as one of the major risk factors for MK, affecting almost five in 10,000 wearers.10 Because of the massive number of contact lens wearers worldwide, the morbidity due to corneal ulcer has public health consequences.11
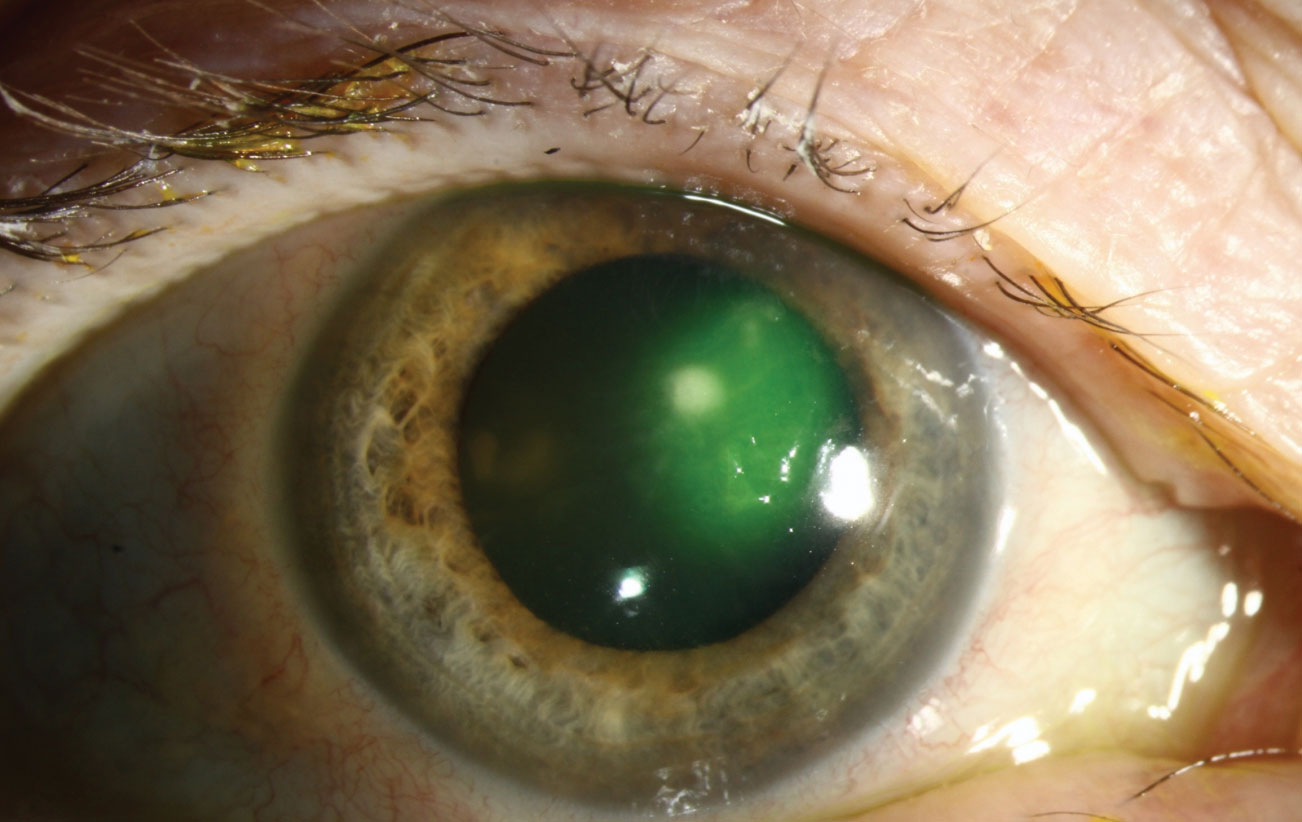 |
| Serratia marcescens (as well as P. aeruginosa and S. aureus) can form biofilms on contact lenses that are resistant to contact lens disinfecting solutions. Photo: Christine W. Sindt, OD. Click image to enlarge. |
Epidemiology
The incidence of CLMK is two to four per 10,000 contact lens wearers per year for daily soft contact lens wearers and 20 per 10,000 for overnight soft contact lens wearers.11-15
Despite the advent of silicone hydrogel lenses that reduce the hypoxic stress to the cornea, especially during overnight wear, the risk for CLMK has not decreased.16 Although daily disposable contact lenses reduce the risk of corneal infiltrative events and severe MK, epidemiological studies have not shown a reduced incidence of MK with daily disposable contact lenses.11,17-19 Of all the lens types, use of gas permeable lenses on a daily wear schedule have the lowest incidence of MK.
Bacteria, especially Pseudomonas species, are the most common pathogens involved in MK, while far fewer—but more severe—infections are caused by Acanthamoeba and fungi.20-23
The incidence of contact lens-related Acanthamoeba keratitis is one to five per one million soft contact lens wearers in Europe and the United States.24 Epidemiological studies have confirmed the use of non-sterile water to clean or store contact lenses and showering or swimming while wearing contact lenses as risk factors for contact lens-related Acanthamoeba keratitis.25-29 Acanthamoeba keratitis has also been linked to both domestic and surface water contamination.25,30,31
Fungal keratitis is rare in contact lens wearers, typically accounting for about 5% of all CLMK.20 Fusarium is a filamentous fungus mainly found in soil and plants.32 Outbreaks of contact lens-related Fusarium keratitis were reported between 2006 and 2007, primarily associated with a specific contact lens disinfection solution (ReNu with MoistureLoc, Bausch + Lomb).33,34 Researchers reported that 60% (10/15) of those with the disease used water to clean contact lens storage cases.35 Following the withdrawal of this disinfection solution from the market, the rates of disease returned to pre-outbreak levels.36
Corneal infiltrative events range from mild asymptomatic corneal infiltrates to severe MK.37 Sterile keratitis is 200 times more prevalent than MK.38
The most prominent contact lens-related risk factor for corneal infiltrative events is extended or overnight lens wear.13,39-42 Research reports an nnnual incidence for severe keratitis for overnight lens wear at 96.4 per 10,000 wearers compared with 6.4 per 10,000 daily lens wearers.42 This suggests an 8.4-fold increase in risk for developing corneal keratitis due to overnight lens wear.
Evidence also suggests a higher rate of contact lens contamination with corresponding increases risk for corneal infiltrative events in lens wearers with two or more years of experience compared with neophytes or those with less than two years of lens wear experience.11,18,19,41,43
Showering with contact lenses or rinsing contact lenses with tap water is associated with an increased risk of contact lens-related corneal infiltrative events.40 Most contact lens disease-causing pathogens are waterborne, so water exposure during lens wear is a serious concern.
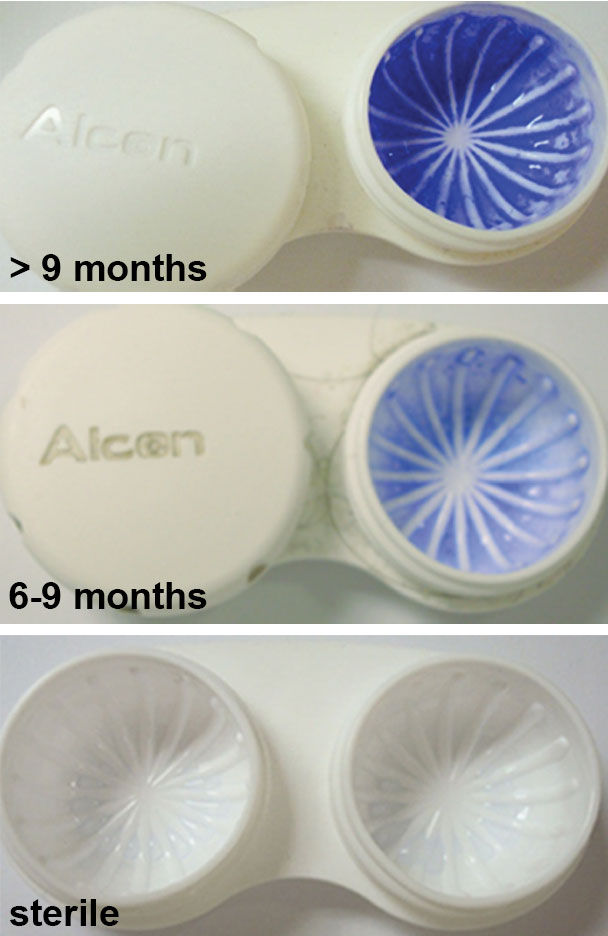 |
| Staining of a contact lens storage case shows the correlation between age and the level of microbial contamination. Staining was evident as early as six months and showed a dramatic increase in intensity beyond nine months. Photo: Danielle M. Robertson, OD, PhD. |
Pathophysiology
Research to understand the mechanisms of ulceration is ongoing.44 Several factors play a crucial role in contact lens-related keratitis:
Bacterial adherence to the lens surface and reduced resistance of the cornea to infection. In bacterial keratitis, bacteria gain access to the corneal stroma through an abnormality or defect in the corneal surface causing an inflammatory response, which results in loss of transparency.45,46 First, penetration of corneal epithelium (more severe than punctate fluorescein staining) needs to take place in the presence of pathogenic levels of bacteria to initiate MK.47 In contact lens wear, hypoxic conditions may increase bacterial binding, compromising corneal integrity and impairing wound healing.11,45,48 Ocular biochemistry changes underneath the contact lens can also predispose the lens wearer to infection.44 Interaction with the contact lens can override the cornea’s defense mechanisms and increase the rate at which the microbes adhere to the ocular surface, leading to MK.44,48,49 The rate of progression of MK depends on the virulence of the offending pathogen and host factors.50
Formation of biofilm on lenses and storage cases. Contact lenses are a fertile surface for bacterial adhesion and biofilm formation.44,48,49 As such, adhesion of bacteria—particularly Staphylococcus epidermis and P. aeruginosa—to contact lenses is a major risk factor.44,49 Contact lens cases are associated with more contamination than lenses or lens care solutions.48,51 Notably, the same strains found in corneal ulcers have been isolated from contact lens cases.51 The level of contamination rate is associated with the age of the lens case.11 The upper rim of the lens case is ideal to harbor gram-negative bacteria due to its air-liquid interface, increasing the likelihood of biofilm formation.51 Contact with this area during lens handling can severely contaminate the lens.51
Resistance of microorganisms to disinfecting systems. Not performing the “rub and rinse” cleaning technique curtails the removal of microbes and creates a carryover effect from lens case to lenses, leading to an increase in microbial virulence and survival rate.44,48,51
Stagnation of tear film behind contact lenses. The presence of debris, toxins and antigens trapped between the contact lens and corneal surface, and their prolonged exposure, can increase the risk of infection.52 This could be the reason for lower CLMK risk with rigid gas permeable lenses, due to higher post-lens tear exchange, compared with soft contact lenses.14,53-56 In addition, epithelial cell proliferation and migration are slower in contact lens wearers, so epithelial cells that reside for a longer time on the corneal surface may initiate inflammation.57-61 Also, tear exchange is drastically reduced in soft contact lens wear.44,45,62 Although the impact of tear exchange is not completely understood, the mean tear elimination rate is 50% less in eyes wearing conventional contact lenses compared with eyes of non-lens wearers.44,48 Incidentally, silicone hydrogels provide better tear exchange than conventional lenses.62
Ocular surfaces of contact lens wearers, compared with non-lens wearers, harbor greater numbers of gram-negative bacteria and fewer numbers of gram-positive bacteria.63,64 Clinicians should consider this evidence and provide adequate prophylactic antibiotic treatment against gram-negative organisms, especially P. aeruginosa, which is the most prevalent gram-negative pathogen in CLMK.63,64
Gram-positive bacteria are the most common organisms in CLMK, especially in temperate climates.10,65 These organisms include coagulase-negative Staphylococcus (including S. epidermidis), S. aureus and Streptococcus pneumoniae. Coagulase-negative Staphylococcus is found on the lid margins and is considered normal flora, but it can become infectious in some instances.66
P. aeruginosa, S. aureus, and Serratia marcescens can form biofilms on contact lenses that are resistant to environmental challenges and contact lens solutions.67-69 P. aeruginosa and S. epidermidis are also found to adhere and replicate on contact lenses in vitro, on both silicone hydrogel and regular hydrogel materials.70
The microbes that are isolated from contact lenses originate from the lid margins, conjunctiva, hands, lens cases, care solutions and the water supply.71-74 Pathogenic organisms are associated with MK, so it is reasonable to assume that if the lid margins, conjunctiva, tear film, contact lens case, care solutions and the patient’s hands are contaminated with these pathogens, the risk of developing MK increases.
Modifiable risk factors. Several modifiable risk factors for MK and corneal inflammatory events are associated with poor compliance. Modifiable risk factors are those that a lens wearer has some control over, as opposed to non-modifiable factors such as age and sex.
The major modifiable risk factors identified in epidemiological studies of CLMK are overnight wear and poor hygiene, including omission of or infrequent lens disinfection, omitted or infrequent lens case cleaning, omission of handwashing before handling lenses and smoking.11,13,75-78 Research estimates that overnight wear and poor hygiene account for about 43% and 33% of attributable risk for developing CLMK.79
Sleeping in contact lenses is another commonly reported contact lens risk behavior and one with a high relative risk for corneal infection.53,80 Research shows sleeping in lenses is a risk factor regardless of lens material and frequency, with even occasional overnight use conferring risk.77
In severe keratitis, contact lens case hygiene (cleaning and replacement) accounts for 63% of the population-attributable risk. In addition, swimming is a risk factor for Acanthamoeba keratitis, and travel is a risk for severe infection, thought to be related to disruption of routine.25,65,81,82
The risk of infection in extended wear is higher with increased wearing time and less experience.11,53,55,83 Wearers considering extended wear should be motivated and aware of this increased risk; however, it is important to balance the risk with other lifestyle risks. Some individuals, such as shift workers and those with busy lifestyles, may feel the convenience of extended wear outweighs the increased risk.
Despite a higher unadjusted incidence rate for daily use of silicone hydrogel contact lenses compared with hydrogel contact lens use, multivariate analyses have not identified lens material as an independent risk factor.11,53
Non-modifiable risk factors. Non-modifiable risk factors include younger age, male gender and socioeconomic class.53,56,78 Systemic risk factors include self-reported poor general health, diabetes and thyroid disease.83,84
More recently, an increased exposure (number of days of wear per week) in daily wear, hypermetropia, obtaining lenses via the internet or mail order and the early period of lens wear have been identified as additional risk factors with contemporary lens types.11,53 Males tend to be more prone to complications in contact lenses, which may be due to increased non-compliance and also a reluctance to seek care.85,86
Genetic differences in contact lens wearers can affect the susceptibility and severity of keratitis. Small mutations (single nucleotide polymorphisms) of interleukins, inflammatory mediators and defensin (an antimicrobial peptide) have been isolated as contributory.87-89 This may mean some people have a degree of innate protection against infection and inflammation when wearing contact lenses, but this protection is much lower than the risk of poor hygiene and overnight wear.
While research shows that risk-taking personality styles are associated with non-compliance in contact lens wearers, no studies indicate whether risk taking is associated with a susceptibility to corneal inflammation and infections.90
There are likely to be many drivers for non-compliance, and not being aware of the risks or understanding the consequences of non-compliance are likely to be key factors among some lens wearers.
Water and Contact Lenses Don’t MixWater exposure during contact lens wear is associated with multiple complications ranging from sterile corneal infiltrates to more severe sight-threatening infections. Clear, unequivocal guidelines/recommendations to avoid all water exposure—including handling contact lenses with wet hands, rinsing/storing contact lenses or storage cases in tap water, and showering with contact lenses—are needed.133 Swimming with contact lenses should be done with protective goggles or using daily disposable contact lenses, which can be discarded immediately after swimming. Active dissemination of these guidelines to contact lens wearers through all stakeholders—including contact lens manufacturers, professional organizations, and contact lens practitioners—is recommended.133 |
Diagnosis
Bacterial keratitis. A substantial inflammatory response along with replicating necrotic cells and microbes form infiltrates that are mostly irregular and focal, surrounded by diffuse inflammation and edema of the cornea.22,91 It is unusual to find a bacterial keratitis with no apparent focal epithelial defect.22,91 In some cases, a focal infiltrate can be absent and an epithelial defect or melting stromal keratitis may be the only signs of infection.22,91 Due to inflammation of the surrounding cornea, causing scattering of light and photophobia, vision can be affected even if the lesion is not central. Other signs such as lid swelling, conjunctival chemosis and anterior chamber reaction are common in bacterial keratitis.91
P. aeruginosa is difficult to neutralize due to its virulent structure, adaptability and high rate of survival under various conditions.44,45 Along with intense immune response, P. aeruginosa also produces enzymes such as protease and elastase, which digest collagen, contributing to corneal melting and perforation.92 Hallmark signs are corneal edema, a ring abscess (defined as a circular infiltrate with a less dense center) associated with larger lesions and presence of hypopyon.93
Acanthamoeba keratitis. Subtle corneal signs with or without symptoms of pain can be found in early infection.94 The early signs include an epitheliopathy with or without a dendritic appearance. Infiltrates running along the nerves from the periphery (perineural infiltrates) are virtually pathologic for Acanthamoeba keratitis, occurring in around 60% of cases.94,95 In later stages of infection, the involvement of central stromal infiltration occurs in around 20% of cases.94 Scleritis occurs in 15% to 20% of cases.94 If scleritis develops, patients often report severe, persistent pain.94 Early diagnosis and prompt, appropriate medical attention improves the prognosis of disease. A delay in effective therapy for more than three weeks will likely worsen the prognosis.96,97
Fungal keratitis. Fungi are opportunistic organisms that do not infect a healthy cornea. However, after trauma and inoculation, fungi can proliferate, leading to tissue damage and the disruption of host defenses.98 Fungi secrete toxins, such as proteases, that aid tissue destruction and allow the fungi to penetrate deep into the cornea. The fungal hyphae and pseudohyphae both form large structures that cannot be fully ingested by polymorphonuclear leukocytes and macrophages. Activation of resident corneal cells and host inflammatory cells that attempt to neutralize the invading organisms adds to the tissue destruction.98
The fungal species that cause keratitis are the yeast, Candida sp., and filamentary fungi such as Aspergillus sp. and Fusarium sp. Contact lens use and trauma are mainly associated with the filamentary fungi, while ocular surface disease is commonly caused by Candida sp.99
Research recently shows microsporidia is a parasitic fungus causes keratoconjunctivitis. It has been found in contact lens wearers, but is usually associated with soil/mud and occurs in immunocompromised patients.100
Differential diagnosis. Distinguishing between Acanthamoeba keratitis and herpes simplex virus (HSV) stromal keratitis can be difficult in the early stages, and around 50% of Acanthamoeba keratitis cases are misdiagnosed as HSV keratitis.101 Epitheliopathy, pseudodendrites and stromal inflammation in Acanthamoeba keratitis are often confused with HSV stromal keratitis. These cases are then treated using corticosteroids. However, use of corticosteroids before the initiation of anti-amoebic drugs is independently associated with a four-fold increased risk of poorer outcomes.101
Differential diagnosis of the clinical signs of fungal keratitis from more common bacterial ulcers can be difficult, especially with yeast fungi such as Candida. Yeast infections tend to present as discrete infiltrates and overlying defects, but unlike most bacterial infections, the onset of yeast-like infections tends to be slow.102 The common features of fungal keratitis cases are serrated ulcer margins with raised slough and a dry textured infiltrate that is usually white or gray (not yellow) and satellite lesions.103 Immune rings (ring infiltrates/Wesley rings) are not pathognomonic for fungal keratitis and can occur in other forms of keratitis, including those caused by Acanthamoeba and bacteria.94,103
Other corneal inflammatory events, such as infiltrative keratitis driven by microbial products and presumed hypersensitivity reactions, typically present with a non-specific inflammatory response that can be local or general.37 Sometimes these can mimic the immune corneal response seen later in the course of adenoviral keratoconjunctivitis.104 Careful history of the redness and symptoms, as well as the swelling of the lymph glands and the more diffuse, fluffy pattern of the infiltrates that are seen in viral conditions, will aid diagnosis.100
In localized sterile inflammation of the cornea, such as marginal keratitis and contact lens-related peripheral ulcer, the redness is usually sectorial corresponding to the corneal lesion and associated with lower pain, a staining diameter greater than the infiltrate diameter (generally <1mm), and possibly a mild anterior chamber reaction. However, a case of infection shows diffuse and more intense redness. In moderate/severe infection, the redness is deeper and the lid as well as anterior chamber are likely to be involved.105
Diagnostic techniques. In cases of suspected MK, corneal scraping is usually the first step to collect samples containing the causative organisms. These samples are then taken for culture or molecular testing using polymerase chain reaction.66 Culture results are available usually within two to seven days.66 Fungi typically take longer than bacteria to grow.
Drug sensitivity testing is performed from the cultured organisms, which is important to guide therapy in nonresponsive cases.66 If antibiotics have been started in nonresponsive cases, the treatment is stopped for 24 hours to maximize the chance of organism recovery.22
Corneal biopsy can be useful for recalcitrant cases.22 The biopsy is deeper than a corneal scrape and can be done freehand or with a trephine. This is particularly useful in fungal keratitis as filamentary fungi may proliferate in the deeper corneal layers, so surface scrapes may not capture the organism. Typically for a biopsy, half the material is sent for microbial culture while the other half is sent to histology for tissue processing.22 Culture tends to be positive in around 50% of cases of clinically presumed bacterial keratitis and slightly higher for Acanthamoeba.22
 |
| Differentiating the clinical signs of fungal keratitis (seen here) from more common bacterial ulcers can be difficult, especially with yeast fungi such as Candida. Photo: Aaron Bronner, OD. Click image to enlarge. |
Management
The goal of treatment is to rapidly eradicate the pathogen, so clinicians should assume CLMK is bacterial unless proven otherwise.106-108 The gold standard treatment for corneal ulceration is fortified antibiotics, such as cefazolin 5% and tobramycin 1.3%, or fourth-generation fluoroquinolones (either ciprofloxacin or ofloxacin) as monotherapy.109,110
Research has observed an alarming trend of increased resistance to antibiotics over the past two decades.111-113 Pathogenic strains such as methicillin-resistant S. aureus (MRSA) and methicillin-resistant S. epidermidis (MRSE) are becoming more prevalent, and many strains show multidrug resistance, including resistance to both earlier and current generation fluoroquinolones.
The most recent addition to topical ocular fluoroquinolones is Besivance (besifloxacin 0.6% ophthalmic suspension, Bausch + Lomb), which decreases resistance due to its unique molecular structure with an increased antibacterial potency.114
Bacterial keratitis. Use of topical antibiotics is the standard treatment for most CLMK. For mild to moderate cases, empirical therapy using a broad‐spectrum fluoroquinolone is generally employed.22 Ciprofloxacin and ofloxacin are the mainstay fluoroquinolone antibiotics. The therapeutic regimen for bacterial keratitis includes use of a cycloplegic agent and frequent use of ciprofloxacin 0.3% antibiotic drops every 15 minutes for four hours, followed by every 30 minutes for four hours, and then every hour around the clock for at least 24 hours. Depending on the severity of the ulcer and the clinical response, ciprofloxacin ointment can be substituted for the drops at a lower frequency during the night after one to two days of therapy. In a severe ulcer, one to two weeks of therapy may be required for a complete therapeutic response.115
The first period is the sterilization phase where the organism is neutralized, followed by the healing phase.22 The sterilization phase usually takes three to five days. Often a dilating agent is used to relax the ciliary body and prevent ciliary spasm, stabilize the blood‐aqueous barrier and to help prevent posterior synechiae. Review is recommended within 24 hours with daily follow-up until improvement is clearly established. In a few days, the eye may be more inflamed due to an inflammatory response to dead organisms, but it is important that there is not a dramatic deterioration in status.22
Some centers tend to treat severe cases with fortified antibiotics that are compounded by an accredited pharmacy. The combination is often a cephalosporin for gram‐positive coverage, such as cephazolin, and an aminoglycoside for gram‐negative activity, either tobramycin or gentamicin. A randomized clinical trial conducted in Australia revealed that monotherapy with ofloxacin 0.3% and moxifloxacin 1% had similar efficacy and safety compared with fortified tobramycin 1.33% and cephazolin 5% antibiotics.116
In many cases, topical steroids are used in the management of bacterial keratitis in an attempt to limit scarring as a lot of the damage in keratitis occurs due to the inflammatory response. Topical steroids also decrease pain and may improve quality of life. A typical regimen introduces steroids once the ulcer begins to re-epithelialize, which is an indication that the antibiotic therapy is effective.
Tapering steroids is essential to avoid a rebound effect. The schedule of steroids, concurrent with antibiotics, might be QID for one week, BID for one week, QD for one week, and then cease. The main concerns for using topical corticosteroids in bacterial keratitis are delayed re‐epithelialization, recurrent infection and increased risk of perforation.8
Acanthamoeba keratitis. There are no currently approved medications for treating Acanthamoeba keratitis. However, biguanides (polyhexamethylene biguanide 0.02% to 0.06% and chlorhexidine 0.02% to 0.2%) and diamidines (propamidine isethionate 0.1% and hexamidine 0.1%) are the most effective cysticidal agents for cases of Acanthamoeba keratitis.117 None of the treatments are licensed for use in the United States but case series have shown good evidence that they are effective in vivo.118
Fungal keratitis. This is often highly invasive, and antifungal agents tend to be fungistatic, leading to prolonged treatment and often surgical intervention.119 Natamycin 5% is usually the initial agent of choice. The Mycotic Ulcer Treatment Trial Phase 1 (MUTT I) found natamycin to be more effective than voriconazole for filamentary fungus, such as Fusarium and Aspergillus.119,120 Also, MUTT II demonstrated that the addition of oral voriconazole to topical natamycin does not improve outcomes.121 Oral voriconazole can also be prescribed for deep fungal ulcers and scleritis, although patients commonly suffer from visual disturbances and will also require liver function tests.122
Anti-fungals are less effective in deeper layers of the cornea. In early phases, rapid progression of fungal keratitis is due to factors relating to organisms such as large fungal inoculum. In later stages, the combination of agent and host factors lead to resistance to anti-fungals.99
Chlorhexidine, an antiseptic for treating Acanthamoeba keratitis, can be used as an alternative to natamycin.121,123 For yeast infections, such as Candida, which is more common in patients with a history of ocular surface disease and in immunosuppressed patients, topical amphotericin B is recommended.119 Echinocandins (such as caspofungin and micafungin) can be added.119 Some evidence suggests that fluoroquinolones may be synergistic with amphotericin.124 Often multiple agents are used to offer maximum coverage.119
Topical steroids should not be used during the treatment of a fungal infection. Corticosteroids induce fungal growth by suppressing ocular immune mechanisms.125 Topical cyclosporine A, however, may be synergistic to fungal therapy because it inhibits filamentary fungal growth.126,127
Therapeutic penetrating keratoplasty. In severe cases of corneal infection, therapeutic penetrating keratoplasty can be considered to prevent the spread of the pathogen to other parts of the eye, especially in filamentary fungal cases. However, this involves the risk of pathogens entering the anterior chamber during surgery.128 Furthermore, corticosteroids used to limit graft rejection may exacerbate the growth of fungus that may remain in the eye following surgery.128 In Acanthamoeba keratitis, therapeutic keratoplasty is limited to impending or perforated corneas, as recurrence often occurs and outcomes are far better following optical keratoplasty in a quiet eye to restore vision.129
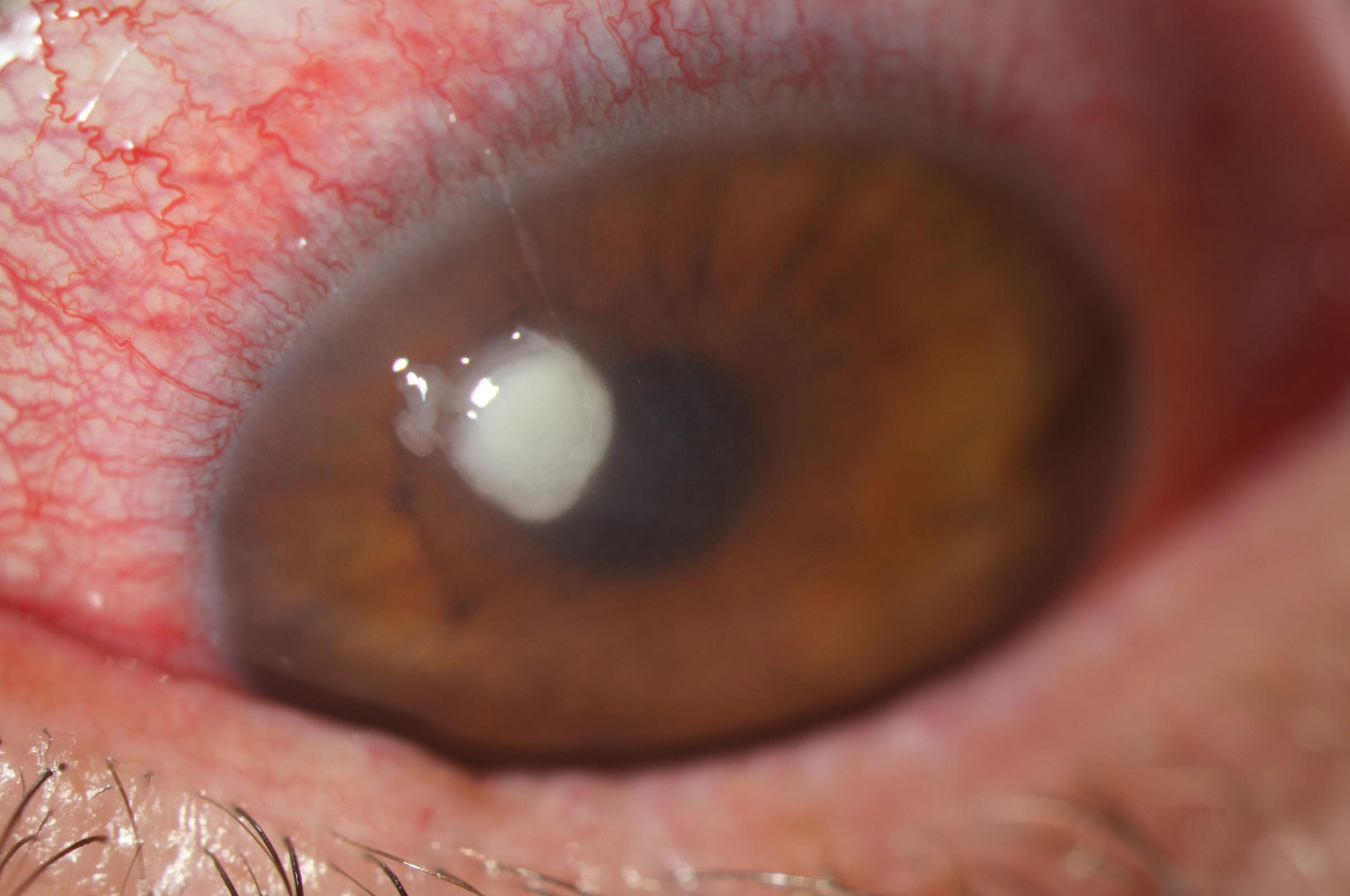 |
| A staphylococcal ulcer typically appears as a discrete infiltrate with well-defined borders and often surrounding edema. Photo: Aaron Bronner, OD. Click image to enlarge. |
Prevention
Contact lens practitioners play a crucial role in the management and education of healthy lens wearers.
Research suggests that 40% to 70% of contact lens wearers are non-compliant.130 Higher rates of complications have been associated with men, teens/young adults, smokers, longer periods of lens wear, lack of hand washing, and internet purchase of lenses.49,51,130,131 Non-compliance with manufacturers’ recommended frequency of replacement of contact lenses is found to be highest among teenagers and wearers of non-silicone hydrogel lenses.130
Contact lens wearers using hydrogen peroxide solution may be more compliant with their lens replacement schedule due to the complex and demanding care regimen.130 Daily disposable lenses were associated with lowest rate of complications in general.130 Better storage lens case designs, frequent replacement of the lens case (at least once in three to six months) and improved hygiene of lens cases may decrease the incidence of corneal ulceration.51
Timely diagnosis and treatment are of paramount importance as early treatment can limit the scarring and vision loss caused by CLMK.46,106,109,132 Treatment delayed by more than 12 hours increases the risk for vision loss.48
Post-marketing surveillance of drugs and devices is important to the health and safety of the general public. Because contact lenses and accompanying lens care solutions are regulated as medical devices by the Food and Drug Administration (FDA) for patient safety, cases of contact lens-related infections should be reported as adverse events to the FDA Safety Information and Adverse Event Reporting Program (www.fda.gov/safety/medwatch). Using the data accumulated in the adverse event reporting program, contact lens stakeholders—industry, regulatory authorities, eye care providers and public health professionals—can work together to determine what improvements can be made to contact lenses, care products, manufacturer guidelines, and labelling.
Dr. Siddireddy is a postdoctoral research fellow specializing in contact lenses, lens care products, dry eye and microbiology at the School of Optometry and Vision Science, University of New South Wales.
1. McLeod SD, LaBree LD, Tayyanipour R, et al. The importance of initial management in the treatment of severe infectious corneal ulcers. Ophthalmology. 1995;102(12):1943-8. 2. McLeod SD, Kolahdouz-Isfahani A, Rostamian K, et al. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103(1):23-8. 3. Benson WH, Lanier JD. Current diagnosis and treatment of corneal ulcers. Curr Opin Ophthalmol. 1998;9(4):45-9. 4. Musch DC, Sugar A, Meyer RF. Demographic and predisposing factors in corneal ulceration. Arch Ophthalmol. 1983;101(10):1545-8. 5. Dart JK. Predisposing factors in microbial keratitis: the significance of contact lens wear. Br J Ophthalmol. 1988;72(12):926-30. 6. Liesegang TJ. Contact lens-related microbial keratitis: Part I: Epidemiology. Cornea. 1997;16(2):125-31. 7. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85(7):842-7. 8. Miedziak AI, Miller MR, Rapuano CJ, et al. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology. 1999;106(6):1166-70; discussion 1171. 9. Vajpayee RB, Dada T, Saxena R, et al. Study of the first contact management profile of cases of infectious keratitis: a hospital-based study. Cornea. 2000;19(1):52-6. 10. Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834-8. 11. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655-62. 12. Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321(12):779-83. 13. Lam DS, Houang E, Fan DS, et al. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye (Lond). 2002;16(5):608-18. 14. Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354(9174):181-5. 15. Seal DV, Kirkness CM, Bennett HG, et al. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22(2):49-57. 16. Papas EB, Vajdic CM, Austen R, Holden BA. High-oxygen-transmissibility soft contact lenses do not induce limbal hyperaemia. Curr Eye Res. 1997;16(9):942-8. 17. Chalmers RL, Hickson-Curran SB, Keay L, et al. Rates of adverse events with hydrogel and silicone hydrogel daily disposable lenses in a large postmarket surveillance registry: the TEMPO Registry. Invest Ophthalmol Vis Sci. 2015;56(1):654-63. 18. Radford CF, Minassian D, Dart JK, et al. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009;116(3):385-92. 19. Steele KR, Szczotka-Flynn L. Epidemiology of contact lens-induced infiltrates: an updated review. Clin Exp Optom. 2017;100(5):473-481. 20. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22-7. 21. Stapleton F, Keay LJ, Sanfilippo PG, et al. Relationship between climate, disease severity, and causative organism for contact lens-associated microbial keratitis in Australia. Am J Ophthalmol. 2007;144(5):690-98. 22. Allan BD, Dart JK. Strategies for the management of microbial keratitis. r J Ophthalmol. 1995;79(8):777-86. 23. Schein OD, Ormerod LD, Barraquer E, et al. Microbiology of contact lens-related keratitis. Cornea. 1989;8(4):281-5. 24. Carnt N, Stapleton F. Strategies for the prevention of contact lens-related Acanthamoeba keratitis: a review. Ophthalmic Physiol Opt. 2016;36(2):77-92. 25. Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86(5):536-42. 26. Fraser MN, Wong Q, Shah L, et al. Characteristics of an Acanthamoeba keratitis outbreak in British Columbia between 2003 and 2007. Ophthalmology. 2012 Jun;119(6):1120-5. 27. Por YM, Mehta JS, Chua JL, et al., Acanthamoeba keratitis associated with contact lens wear in Singapore. Am J Ophthalmol. 2009;148(1):7-12. 28. Kilvington S, Gray T, Dart J, et al. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004;45(1):165-9. 29. Butler TK, Males JJ, Robinson LP, et al. Six-year review of Acanthamoeba keratitis in New South Wales, Australia: 1997-2002. Clin Exp Ophthalmol. 2005;33(1):41-6. 30. Ji WT, Hsu BM, Chang TY, et al. Surveillance and evaluation of the infection risk of free-living amoebae and Legionella in different aquatic environments. Sci Total Environ. 2014;499:212-9. 31. Joslin CE, Tu EY, McMahon TT, et al. Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol. 2006;142(2):212-7. 32. Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7(4):479-504. 33. Saw SM, Ooi PL, Tan DT, et al. Risk factors for contact lens-related fusarium keratitis: a case-control study in Singapore. Arch Ophthalmol. 2007;125(5):611-7. 34. Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296(8):953-63. 35. Gorscak JJ, Ayres BD, Bhagat N, et al. An outbreak of Fusarium keratitis associated with contact lens use in the northeastern United States. Cornea. 2007;26(10):1187-94. 36. Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117(12):2263-7. 37. Sweeney DF, Jalbert I, Covey M, et al. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea. 2003;22(5):435-42. 38. Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84(4):257-72. 39. Cutter GR, Chalmers RL, Roseman M. The clinical presentation, prevalence, and risk factors of focal corneal infiltrates in soft contact lens wearers. CLAO J. 1996;22(1):30-7. 40. Richdale K, Lam DY, Wagner H, et al. Case-control pilot study of soft contact lens wearers with corneal infiltrative events and healthy controls. Invest Ophthalmol Vis Sci. 2016;57(1):47-55. 41. Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011;52(9):6690-6. 42. Morgan PB, Efron N, Hill EA, et al. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol. 2005;89(4):430-6. 43. Wu YT, Willcox MD, Stapleton F. The effect of contact lens hygiene behavior on lens case contamination. Optom Vis Sci. 2015;92(2):167-74. 44. Fleiszig SM, Evans DJ. The pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci. 2010;87(4):225-32. 45. Fleiszig SM. The pathogenesis of contact lens-related keratitis. Optom Vis Sci. 2006;83(12):866-73. 46. Cornea/External Disease Preferred Practice Pattern Panel. Bacterial Keratitis Preferred Practice Pattern. American Academy of Ophthalmology. 2018. www.aaojournal.org/article/S0161-6420(18)32644-7/pdf. 47. Willcox MD. Which is more important to the initiation of contact lens related microbial keratitis, trauma to the ocular surface or bacterial pathogenic factors? Clin Exp Optom. 2006;89(5):277-9. 48. Musa F, Tailor R, Gao A, et al. Contact lens-related microbial keratitis in deployed British military personnel. Br J Ophthalmol. 2010;94(8):988-93. 49. Giraldez MJ, Resua CG, Lira M, et al. Contact lens hydrophobicity and roughness effects on bacterial adhesion. Optom Vis Sci. 2010;87(6):E426-31. 50. Ahn M, Yoon KC, Ryu SK, et al. Clinical aspects and prognosis of mixed microbial (bacterial and fungal) keratitis. Cornea. 2011;30(4):409-13. 51. Wu YT, Zhu H, Harmis NY, et al. Profile and frequency of microbial contamination of contact lens cases. Optom Vis Sci. 2010;87(3):E152-8. 52. Zantos S. Corneal infiltrates, debris and microcysts. J Am Optom Assoc. 1984;55:196-8. 53. Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115(10):1647-54. 54. Nilsson SE, Montan PG. The annualized incidence of contact lens induced keratitis in Sweden and its relation to lens type and wear schedule: results of a 3-month prospective study. CLAO J. 1994;20(4):225-30. 55. Stapleton F, Dart JK, Minassian D. Risk factors with contact lens related suppurative keratitis. CLAO J. 1993;19(4):204-10. 56. Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338(8768):650-3. 57. Ladage PM, Ren DH, Petroll WM, et al. Effects of eyelid closure and disposable and silicone hydrogel extended contact lens wear on rabbit corneal epithelial proliferation. Invest Ophthalmol Vis Sci. 2003;44(5):1843-9. 58. Ladage PM, Jester JV, Petroll WM, et al. Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Invest Ophthalmol Vis Sci. 2003;44(3):1056-63. 59. O’Leary DJ, Madgewick R, Wallace J, Ang J. Size and number of epithelial cells washed from the cornea after contact lens wear. Optom Vis Sci. 1998;75(9):692-6. 60. Ren DH, Petroll WM, Jester JV, et al. The relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear. CLAO J. 1999;25(2):80-100. 61. Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108(7):1279-88. 62. Paugh JR, Stapleton F, Keay L, Ho A. Tear exchange under hydrogel contact lenses: methodological considerations. Invest Ophthalmol Vis Sci. 2001;42(12):2813-20. 63. Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye (Lond). 1991;5(Pt 1):70-4. 64. Høvding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol. 1981;59(3):387-401. 65. Edwards K, Keay L, Naduvilath T, et al. Characteristics of and risk factors for contact lens-related microbial keratitis in a tertiary referral hospital. Eye (Lond). 2009;23(1):153-60. 66. Jones DB, Liesegang TJ, Robinson NM. Laboratory diagnosis of ocular infections. Am Society for Microbiology, Washington, DC; 1981. 67. Willcox MD, Harmis N, Cowell BA, et al. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials. 2001;22(24):3235-47. 68. Szczotka-Flynn L, Lass JH, Sethi A, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51(11):5421-30. 69. McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JK. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84(5):827-38. 70. Kodjikian L, Casoli-Bergeron E, Malet F, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):267-73. 71. Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010;36(2):116-29. 72. Jiang Y, Jacobs M, Bajaksouzian S, et al. Risk factors for microbial bioburden during daily wear of silicone hydrogel contact lenses. Eye Contact Lens. 2014;40(3):148-56. 73. Wu YT, Willcox M, Zhu H, Stapleton F. Contact lens hygiene compliance and lens case contamination: A review. Cont Lens Anterior Eye. 2015;38(5):307-16. 74. Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86(5):536-42. 75. Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338(8768):650-3. 76. Radford CF, Minassian DC, Dart JK. Disposable contact lens use as a risk factor for microbial keratitis. Br J Ophthalmol. 1998;82(11):1272-5. 77. Stapleton F, Edwards K, Keay L, et al. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology. 2012;119(8):1516-21. 78. Morgan PB, Efron N, Brennan NA, et al. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Invest Ophthalmol Vis Sci. 2005;46(9):3136-43. 79. Keay L, Stapleton F. Development and evaluation of evidence-based guidelines on contact lens-related microbial keratitis. Cont Lens Anterior Eye. 2008;31(1):3-12. 80. Cope JR, Collier SA, Nethercut H, et al. Risk behaviors for contact lens-related eye infections among adults and adolescents—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(32):841-45. 81. Radford CF, Lehmann OJ, Dart JK. Acanthamoeba keratitis: multicentre survey in England 1992-6. Br J Ophthalmol. 1998;82(12):1387-92. 82. Seal DV, Kirkness CM, Bennett HG, Peterson M. Acanthamoeba keratitis in Scotland: risk factors for contact lens wearers. Cont Lens Anterior Eye. 1999;22(2):58-68. 83. Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. N Engl J Med. 1989;321(12):773-8. 84. Keay L, Edwards K, Stapleton F. Signs, symptoms and comorbidities in contact lens-related microbial keratitis. Optom Vis Sci. 2009;86(7):803-9. 85. Phillips SP. Defining and measuring gender: a social determinant of health whose time has come. Int J Equity Health. 2005;4:11. 86. Brett KM, Burt CW. Utilization of ambulatory medical care by women; United States, 1997-1998. Vital Health Stat 13. 2001;(149):1-46. 87. Keijser S, Kurreeman FA, de Keizer RJ, et al. IL-10 promotor haplotypes associated with susceptibility to and severity of bacterial corneal ulcers. Exp Eye Res. 2009;88(6):1124-8. 88. Carnt NA, Willcox MD, Hau S, et al. Association of single nucleotide polymorphisms of interleukins-1β, -6, and -12B with contact lens keratitis susceptibility and severity. Ophthalmology. 2012;119(7):1320-7. 89. Carnt N, Robaei D, Watson SL, et al. The impact of topical corticosteroids used in conjunction with antiamoebic therapy on the outcome of acanthamoeba keratitis. Ophthalmology. 2016;123(5):984-90. 90. Carnt N, Keay L, Willcox M, et al. Higher risk taking propensity of contact lens wearers is associated with less compliance. Cont Lens Anterior Eye. 2011;34(5):202-6. 91. Carnt N, Samarawickrama C, White A, Stapleton F. The diagnosis and management of contact lens-related microbial keratitis. Clin Exp Optom. 2017;100(5):482-93. 92. Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84(4):273-8. 93. Oka N, Suzuki T, Ishikawa E, et al. Relationship of virulence factors and clinical features in keratitis caused by Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 2015;56(11):6892-8. 94. Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487-499.e2. 95. Robbie SJ, Vega FA, Tint NL, et al. Perineural infiltrates in Pseudomonas keratitis. J Cataract Refract Surg. 2013;39(11):1764-7. 96. Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):648-53. 97. Tu EY, Joslin CE, Sugar J, et al. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115(11):1998-2003. 98. Abad JC, Foster CS. Fungal keratitis. Int Ophthalmol Clin. 1996;36(3):1-15. 99. Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730-97. 100. Loh RS, Chan CM, Ti SE, et al. Emerging prevalence of microsporidial keratitis in Singapore: epidemiology, clinical features, and management. Ophthalmology. 2009;116(12):2348-53. 101. Robaei D, Carnt N, Minassian DC, Dart JK. The impact of topical corticosteroid use before diagnosis on the outcome of Acanthamoeba keratitis. Ophthalmology. 2014;121(7):1383-8. 102. Chang HY, Chodosh J. Diagnostic and therapeutic considerations in fungal keratitis. Int Ophthalmol Clin. 2011;51(4):33-42. 103. Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89(12):1554-8. 104. Kaufman HE. Adenovirus advances: new diagnostic and therapeutic options. Curr Opin Ophthalmol. 2011;22(4):290-3. 105. Aasuri MK, Venkata N, Kumar VM. Differential diagnosis of microbial keratitis and contact lens-induced peripheral ulcer. Eye Contact Lens. 2003;29(1 Suppl):S60-2; discussion S83-4, S192-4. 106. Garg P. Diagnosis of microbial keratitis. Br J Ophthalmol. 2010;94(8):961-2. 107. Kaiser PK, Friedman NJ, Pineda R, et al. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 4th ed. London: Saunders; 2014. 108. Ehlers JP, Shah CP. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. Philadelphia: Lippincott Williams & Wilkins; 2008. 109. Shah VM, Tandon R, Satpathy G, et al. Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea. 2010;29(7):751-7. 110. Chawla B, Agarwal P, Tandon R, et al. In vitro susceptibility of bacterial keratitis isolates to fourth-generation fluoroquinolones. Eur J Ophthalmol. 2010;20(2):300-5. 111. Miller D, Flynn PM, Scott IU, et al. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Ophthalmol. 2006;124(4):479-83. 112. Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249(1):111-9. 113. Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance among ocular pathogens in the united states: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance study. JAMA Ophthalmol. 2015;133(12):1445-54. 114. Mah FS, Sanfilippo CM. Besifloxacin: Efficacy and safety in treatment and prevention of ocular bacterial infections. Ophthalmol Ther. 2016;5(1):1-20. 115. Katz HR, Morris S, Woolridge R. At Issue: Cycloplegics, fluoroquinolones primary treatment for bacterial keratitis. Primary Care Optometry News. 2001;6(4). 116. Constantinou M, Daniell M, Snibson GR, et al. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114(9):1622-9. 117. Elder MJ, Kilvington S, Dart JK. A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1994;35(3):1059-64. 118. Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487-499.e2. 119. Ng JK, Fraunfelder FW, Winthrop KL. Review and update on the epidemiology, clinical presentation, diagnosis, and treatment of fungal keratitis. Curr Fungal Infect Rep. 2013;7(4):293-300. 120. Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422-9. 121. Prajna NV, Krishnan T, Rajaraman R, et al; Mycotic Ulcer Treatment Trial II Group. Effect of oral voriconazole on fungal keratitis in the mycotic ulcer treatment trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1365-72. 122. Zrenner E, Tomaszewski K, Hamlin J, et al. Effects of multiple doses of voriconazole on the vision of healthy volunteers: a double-blind, placebo-controlled study. Ophthalmic Res. 2014;52(1):43-52. 123. Rahman MR, Minassian DC, Srinivasan M, et al. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiol. 1997;4(3):141-9. 124. Stergiopoulou T, Meletiadis J, Sein T, et al. Isobolographic analysis of pharmacodynamic interactions between antifungal agents and ciprofloxacin against Candida albicans and Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52(6):2196-204. 125. Stern GA, Buttross M. Use of corticosteroids in combination with antimicrobial drugs in the treatment of infectious corneal disease. Ophthalmology. 1991;98(6):847-53. 126. Cardenas ME, Cruz MC, Del Poeta M, et al. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin Microbiol Rev. 1999;12(4):583-611. 127. Perry HD, Doshi SJ, Donnenfeld ED, Bai GS. Topical cyclosporin A in the management of therapeutic keratoplasty for mycotic keratitis. Cornea. 2002;21(2):161-3. 128. Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209-218. 129. Robaei D, Carnt N, Minassian DC, Dart JK. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: risk factors, outcomes, and summary of the literature. Ophthalmology. 2015;122(1):17-24. 130. Yeung KK, Forister JF, Forister EF, et al. Compliance with soft contact lens replacement schedules and associated contact lens-related ocular complications: The UCLA Contact Lens Study. Optometry. 2010;81(11):598-607. 131. Chalmers RL, Keay L, Long B, et al. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci. 2010;87(10):725-35. 132. Huerva V, Sanchez MC. Refractive outcome after severe Pseudomonas aeruginosa keratitis. Optom Vis Sci. 2011;88(4):E548-52. 133. Arshad M, Carnt N, Tan J, et al. Water exposure and the risk of contact lens-related disease. Cornea. 2019;38(6):791-797. |
