The ABCs of Radiologic Testing
Clinicians should be prepared to order CT and MRI should the need arise. Patients’ lives may count on it.
By Jason Fliegel, OD, Traci Seng, OD, Sara Weidmayer, OD, and Kathy Lewis, OD
Release Date: October 15, 2018
Expiration Date: October 15, 2021
Goal Statement: Clinicians often face several roadblocks when considering radiologic imaging, including unfamiliarity with the available tests, uncertainty as to what type of modality is appropriate and even the possibility of unwanted adverse effects. However, CT and MRI are often valuable to help uncover the appropriate diagnosis and guide treatment—and ODs need to be prepared to order these tests to improve their diagnostic accuracy.
Faculty/Editorial Board: Jason Fliegel, OD, Traci Seng, OD, Sara Weidmayer, OD, and Kathy Lewis, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 59487-PD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The authors have no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Catherine Manthorp and Mark De Leon all have no relationships to disclose.
The pursuit of diagnostic imaging during the course of optometric patient care can be an intimidating clinical decision. Optometrists often face several roadblocks, including unfamiliarity with available imaging options, uncertainty as to what type of modality is appropriate for a certain case and even the possibility of unwanted adverse effects.
Nonetheless, common radiological procedures such as computed tomography (CT) and magnetic resonance imaging (MRI) are often necessary to obtain the appropriate diagnosis and guide treatment. Here, we discuss the indications for, and precautions associated with, specific types of imaging to help clarify several common concerns that often hinder their use in optometric practice.
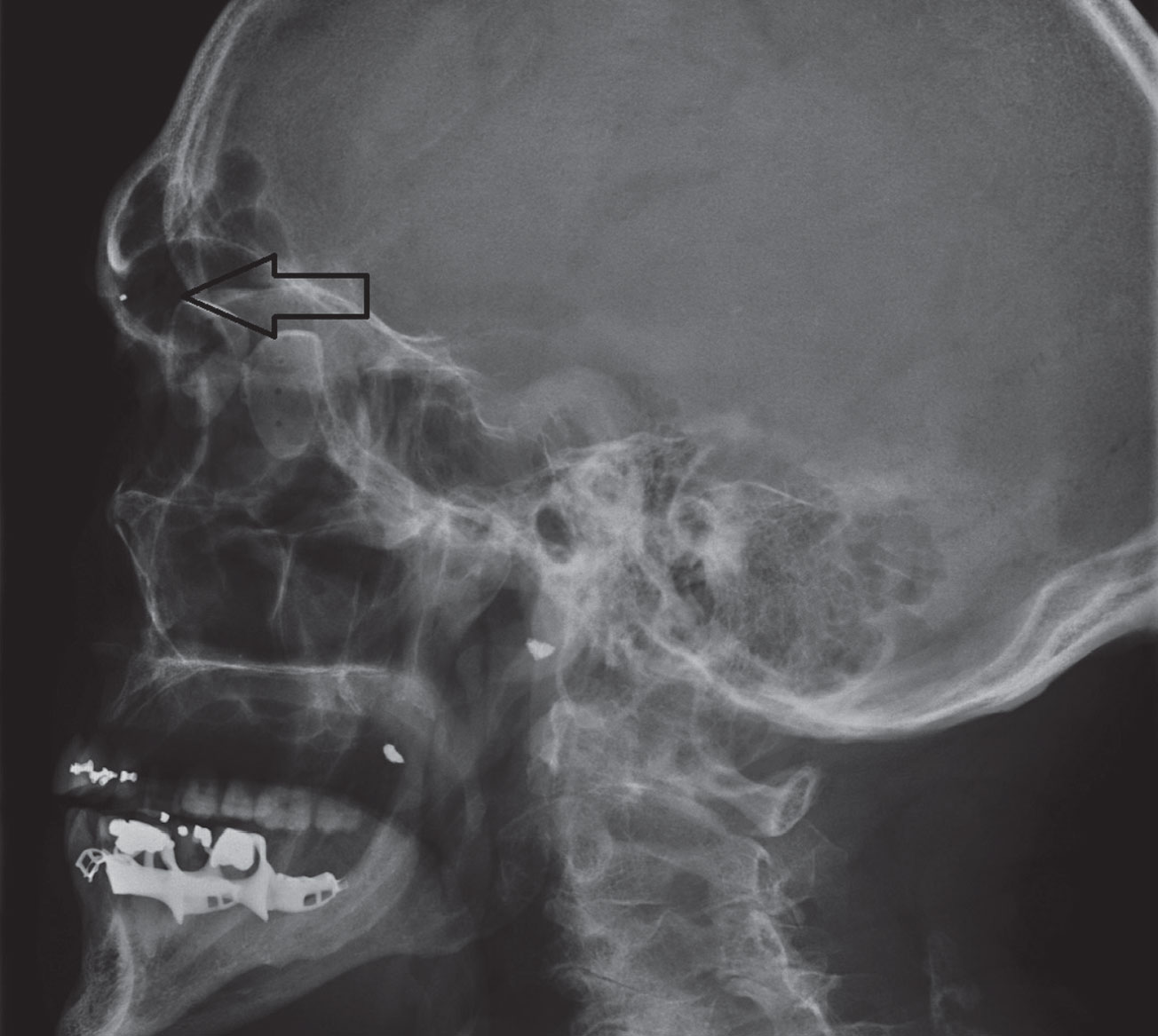
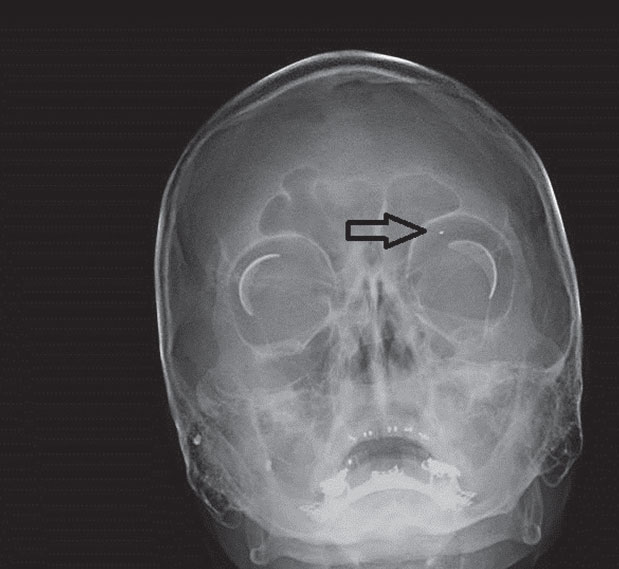 |
| Fig. 1. These radiographic x-ray coronal (above) and sagittal (right) images demonstrate two small metallic shrapnel fragments overlying the right mandible and a smaller shrapnel fragment within the left orbit. Click right image to enlarge. |
 |
Conventional Radiography
The x-ray, a type of electromagnetic energy and source of ionizing radiation, can pass through substances, such as bone, that are typically impenetrable to light.1,2 In conventional radiography, x-rays are passed through the area of focus.1,2 The amount of x-ray attenuation reflects the various densities of the involved structures and is typically captured in a film or digital format, resulting in the familiar two-dimensional image.1 Structural densities that can be visualized with x-ray include air, bone, fat, soft tissue and metal.3
A common concern and disadvantage of undergoing x-ray imaging is the exposure to ionizing radiation. However, the amount of radiation used in conventional radiography is significantly less than that of CT, which also uses x-ray, and therefore carries less risk for developing a radiation-related disease such as cancer.4
In eye care, the use for conventional radiography is limited. Orbital radiographs are mainly used to screen patients who have a history or possible history of intraocular or intraorbital foreign bodies prior to undergoing MRI due to the potential risk for mobilization of ferromagnetic foreign bodies (Figure 1).5,6 One study suggests 0.27% of the population has intraorbital metallic foreign bodies, compared with 2.5% of those with an occupational history of metalworking or prior metallic foreign body trauma.7 Furthermore, the American College of Radiology recommends “all patients who have a history of orbit trauma by a potential ferromagnetic foreign body for which they sought medical attention are to have their orbits cleared by either plain x-ray orbit films (two views) or by a radiologist’s review and assessment of contiguous cut prior CT or MR images (obtained since the suspected traumatic event) if possible.”8
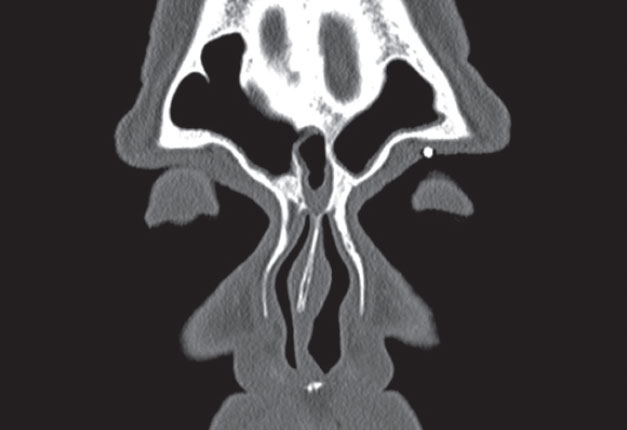
Conventional radiography, however, should not be used to fully exclude a potential foreign body if highly suspected or in cases of recent ocular or orbital trauma; CT is recommended for these clinical situations (Figure 2).6,9
Conventional x-ray may also assist in the diagnosis of systemic conditions that often present with ocular manifestations. For example, in patients presenting with uveitis, chest radiography may reveal bilateral hilar lymphadenopathy consistent with sarcoidosis, whereas the presence of erosion and sclerosis of the sacroiliac joint are common radiographic findings with ankylosing spondylitis (Figure 3).10,11
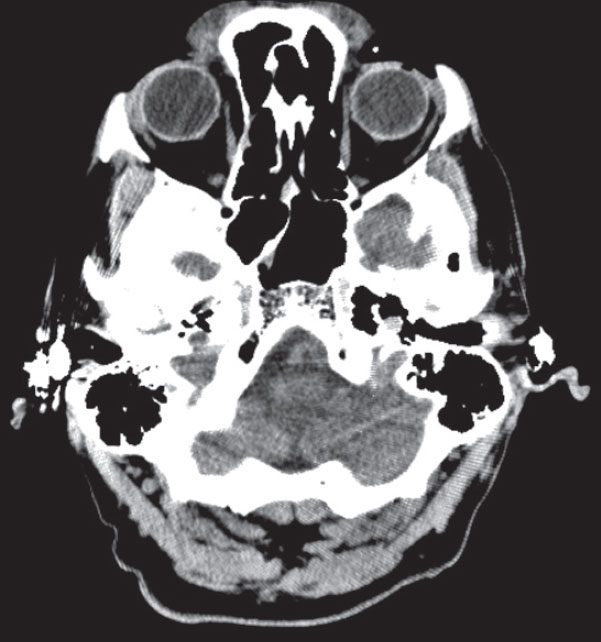 |
| Fig. 2. These axial (above) and coronal (left) CT images demonstrate the same shrapnel fragment in Figure 1 at the margin of the left superior orbital rim. |
 |
Computed Tomography
With this modality, an x-ray tube is rotated around the area of focus, after which the amount of x-ray attenuation is measured by opposing detectors.12-14 The degree of attenuation, dependent on the density of the tissues or materials in question, is represented as a numerical value known as Hounsfield Unit (HU).12,13 Scan density is then visualized as a 2D or 3D cross-sectional image after Hounsfield Units are assigned to corresponding pixels, each of which have individual gray-scale values ranging from 1 (black) to 256 (white).12,13,15,16
The advantages of CT over other imaging modalities include widespread availability, relative cost effectiveness and timely scan completion.12,15 Clinically, CT’s ability to provide superior detail of bone abnormalities, calcification, bony involvement from a soft tissue mass and metallic foreign bodies is outstanding.6,15,17 CT is especially sensitive at detecting fresh blood, thus making it the preferred imaging option for suspected acute intracranial hemorrhagic conditions.6,13,15,18 When iodinated contrast medium is used in conjunction with CT, both specificity and sensitivity of the scan for various pathologic conditions can increase.12,13,15 Improved visualization of a breakdown of the blood-brain barrier, as well as infectious, inflammatory and neoplastic conditions are a few indications that may warrant the use of contrast.6,18
As with conventional radiography, exposure to ionizing radiation is a common concern with CT, which could potentially cause damage at a cellular level and increase the risk for cancer.4,19 The millisievert (mSv) is the unit of measurement used to quantify the effective dose of radiation and risk associated with exposure.4 In the United States, individuals typically encounter an annual effective dose of around 3.1mSv from naturally-occurring background radiation exposure.20 By comparison, CT of the head reveals an effective dose of 2mSv when done without contrast and 4mSv when done with and without contrast, which is approximately equivalent to eight and 16 months, respectively, of background radiation exposure.21
An x-ray of the chest has an effective dose of only 0.1mSv.21 Regardless, the lifetime risk of death from cancer following a single head CT remains low (less than 0.08%), although the risk is much higher when the scan is completed from birth through age 15.22 Cumulative radiation dose is important, as research shows CT could increase the lifetime risk of cancer in patients who have received multiple scans.23
Given these common concerns, clinicians should take special precautions when considering imaging for certain patient populations:
Pregnancy. CT should be deferred if at all possible in pregnant women, as studies suggest that exposing a developing fetus to ionizing radiation may increase the risk of developing childhood cancers and possibly lead to malformations, developmental disturbances and even loss of pregnancy.17,24-27 Characteristics that influence the amount of fetal radiation exposure include scan location (e.g., head vs. abdomen) and slice thickness.28 A pregnancy test should be considered prior to CT for women of child-bearing age.6
Children. Increased organ radio-sensitivity, smaller body size and longer lifespan may increase the risk for radiation-related cancers in children.4,29 CT is generally avoided unless absolutely indicated, such as in cases of orbital or head trauma where CT remains the preferred method of imaging.6
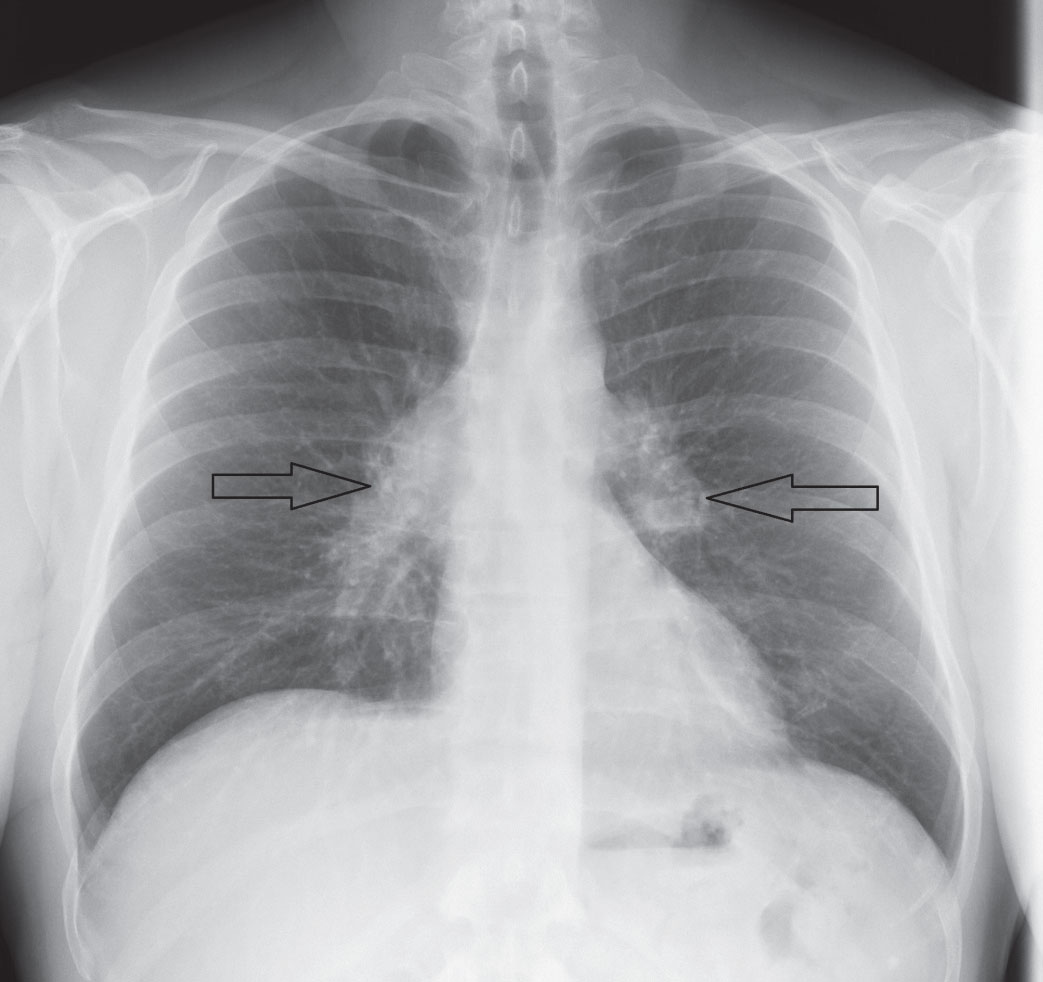 |
| Fig. 3. A chest x-ray reveals sarcoid-associated bilateral hilar lymphadenopathy (arrows). Click image to enlarge. |
Adding Contrast
The use of iodinated contrast media is not without the possibility of complication; adverse reactions can be mild to severe and include nausea, vomiting, urticaria, bronchospasm, hypotension, anaphylactic shock and even cardiac arrest.14 Risk factors for an adverse effect include a prior allergic reaction to contrast, asthma, severe cardiac disease, renal insufficiency and procedural anxiety.30 Clinicians should consider these four precautions prior to ordering CT with contrast:
1. Contrast may be contraindicated in patients who have exhibited an allergic or physiologic reaction to the use of a previous contrast agent. However, in those patients where CT with contrast is a necessity and other imaging modalities are not the preferred option, medications such as corticosteroids (e.g., “steroid prep”) and diphenhydramine are typically given prior to the use of contrast to reduce the risk of a possible adverse reaction.14,15,31
2. Contrast-induced nephropathy is a concern for those with a prior history of impaired renal function or acute kidney injury.31 Therefore, the use of iodinated contrast should be discussed with the appropriate specialties to decide if it is appropriate and to ensure the proper hydration protocol can take place prior to the procedure.14,31 Additionally, patients taking metformin with a prior history of renal dysfunction may be asked to discontinue the medication 48 hours prior to the use of contrast media due to the increased risk for lactic acidosis.14,30,31
3. While children and women who are pregnant or breastfeeding have no absolute contraindications with iodinated contrast, adverse events such as allergic-like reactions and nephrotoxicity can also occur; its use is generally avoided if possible.30-32
4. Patients with untreated hyperthyroidism are contraindicated from receiving iodinated contrast due to the risk for thyrotoxicosis.14,32
Imaging VariationsWhile conventional catheter cerebral angiography via the femoral artery remains the standard, magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) are typically initially used to assist in the detection of suspected intracranial vascular conditions, such as aneurysms or dissections.6,18,42 MRA and CTA can be advantageous in comparison with catheter angiography due to their availability, timeliness of completion and their noninvasiveness.18,42 While CTA allows for more expedient and generally more sensitive results, iodinated contrast medium and exposure to radiation may make MRA necessary in certain clinical situations.6,13,18 Suspicion of cerebral venous sinus thrombosis may warrant magnetic resonance venography (MRV) or computed tomographic venography (CRV) to exclude it as causal for papilledema.42 Advantages and disadvantages similar to that of MRA and CTA remain with both MRV and CTV, and clinicians must use their discretion when deciding which imaging modality would best serve the patient.15,42 |
Order and Interpret CT
Clinicians should clearly communicate the patient’s clinical history, suspected diagnoses and potential contraindications or precautions to the radiology department in the CT order.17
Within eye care, CT is typically used for conditions involving the orbit and ordered accordingly; however, additional views (e.g., head, neck or both) may be necessary depending on the clinical situation. Scans involving the orbit are typically ordered without contrast or with and without contrast in the axial and coronal planes with a slice thickness of between 1mm and 3mm, depending on recommended protocol for the suspected condition.6,9,18,33 Clinicians who are uncertain of the proper protocol should consult with the radiologist.
Although the radiologist will complete the CT interpretation and report, optometrists should have a familiar knowledge of normal vs. abnormal anatomy for additional review. The first step in interpreting the results is understanding the units of measure. An HU of 0 is equal to water, -1,000 is equal to air and bone approaches +1,000.12,14 Following conversion of all HUs to the corresponding greyscale image, denser tissues such as bone will appear white, while air will appear black.12,15 Tissue or substances such as fat, muscle, blood and contrast medium typically exhibit a range of HU values and are represented accordingly on the greyscale image.14,34
Abnormalities are typically described in comparison with surrounding structures as isodense (comparable with the brain), hyperdense (bone, acute blood, calcification), hypodense (edema, infarction) or enhanced with contrast (inflammation, neoplasms).35 The report should also include a description of how the area of concern is impacting any surrounding structures (e.g., producing midline shift, mass effect, etc.).14 Radiologists will also often use bone windows to further isolate areas of bony detail concern, rather than using the standard soft tissue setting (Figure 4).6,36
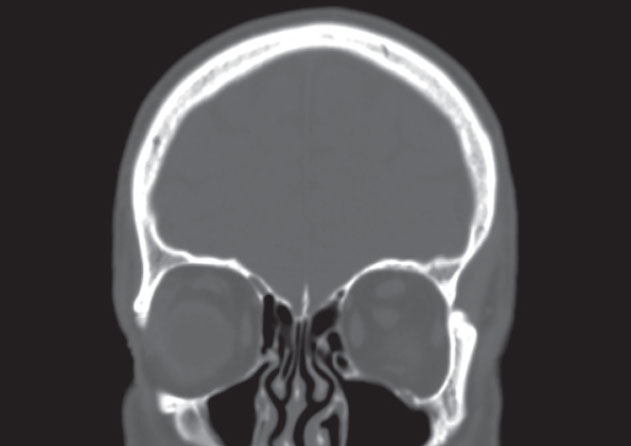 |
| Fig. 4. Radiologists often use bone window settings to improve bony detail when evaluating conditions such as suspected orbital fractures or bony erosion from metastatic tumors. |
The When and Why of CT
Clinicians can use this imaging technique to better assess pertinent structures in any number of clinical presentations:
Orbital trauma. Clinical signs and symptoms that indicate the need for CT following recent orbital trauma include decreased visual acuity or field of vision, new-onset afferent pupillary defect, diplopia, restricted extraocular motility, pain on eye movement, proptosis, lid edema, ecchymosis, severe subconjunctival hemorrhage and optic disc edema.6,9 Any concern for conditions such as an orbital wall fracture, orbital hemorrhage or traumatic optic neuropathy warrant CT of the orbit and possibly the head, face or both, typically without contrast with a thin slice thickness (1mm to 3mm, depending on the recommended protocol) in the axial and coronal planes.6,9,37
Extraocular muscles. Proptosis, ocular motility restriction, eyelid retraction, periorbital edema and ocular surface involvement are signs of Graves’ ophthalmopathy.6,9,37 While MRI with gadolinium contrast may also be used to evaluate orbital involvement from Graves’, axial and coronal views of the orbit using CT without contrast is generally appropriate to visualize enlargement of the extraocular muscles without tendon involvement, which is typical of this condition.6,9 While iodinated contrast with CT may allow for enhancement of the involved muscles, it is usually not recommended out of concern for contrast-related thyrotoxicosis.13,17,38
Orbital disease. Proptosis, diplopia, swelling of the eyelids, pain and visual decrease may present in cases of orbital disease such as inflammatory orbital pseudotumor, orbital cellulitis or orbital abscess, meningiomas, mucoceles, orbital lymphoma and orbital varix.6,9,15,33
While MRI may be preferred in certain instances, CT may still provide valuable insight. CT of the orbit (possibly with specified inclusion of the paranasal sinuses) and other pertinent structures such as the brain, with and without contrast in the axial and coronal planes is appropriate for suspected inflammatory, infectious or neoplastic orbital conditions.6,9,33,37 CT is especially useful in detecting bony erosion associated with tumors such as metastatic neuroblastoma in children or metastatic and lymphoid tumors in adults.6,9,15 The modality is also exceptional at detecting calcification within a tumor.6,15,18 This is particularly important in children with retinoblastoma, as 95% of those cases reveal calcification and can assist in diagnosis of this life-threatening condition.15,39
Intraocular, intraorbital foreign bodies. CT is recommended when concern exists for a penetrating injury following a history of high-speed projectile foreign body trauma to or around the eye. Clinicians should obtain axial and coronal views without contrast of the orbit and, possibly, the brain with a slice thickness of less than 1mm, if possible.6,9,33 Objects composed of metal and glass are best observed with CT, whereas wood or vegetative matter may be better detected with B-scan, provided there are no contraindications for the procedure.6,9,35
 |
| Fig. 4. Radiologists often use bone window settings to improve bony detail when evaluating conditions such as suspected orbital fractures or bony erosion from metastatic tumors. Click image to enlarge. |
Magnetic Resonance Imaging
This technique is based on the principle that the nuclei of atoms containing an odd number of protons, neutrons or both become polarized or aligned when placed in a static magnetic field.40-42 For imaging human tissue, the hydrogen atom is primarily responsible for these magnetic properties.40,42 In addition to a strong static magnetic field, a transitory oscillating radiofrequency field (RF) is generated that, when switched off, causes the magnetic nuclei to emit nuclear magnetic resonance signals, which are then processed to form an MRI image.40,41
MRI techniques and images are characterized according to time, specifically T1-weighted (T1W) and T2-weighted (T2W). These time constants correspond to the behavior of protons responding to the magnetic field and RF. The RF field causes excitation of the protons, and after the RF field is terminated, the protons realign to their original location, known as relaxation. Longitudinal relaxation, or spin-lattice, is referred to as T1W, while transverse relaxation, or spin-spin, is referred to as T2W. T1W and T2W relaxation occur simultaneously; however, T2W relaxation is completed much more rapidly.40-42 Unlike CT, there is no radiation exposure with MRI.
Image appearance of tissues with MRI depends on the proton density of the structures being imaged, timing of the RF pulse and sampling, fluid flow and electron clouds surrounding the nuclei, which may shield the atom from the applied magnetic field.40 T1W images of tissues with shorter T1W relaxation times, such as fat and blood, appear hyperintense (brighter) compared with those with longer times such as water, vitreous and cerebrospinal fluid (CSF).15,41
In T2W images, tissues with longer T2W relaxation appear hyperintense compared with those with shorter times such as blood products (Figure 5).15,40,41 In general, normal anatomy is best demonstrated on T1W images, whereas intracranial pathology is better imaged with T2W (Figure 6).43 Both are included as part of a standard MRI examination.43
Although CT neuroimaging is more common, MRI is typically more appropriate for assessment of neuro-ophthalmic diseases. In general, MRI is superior to CT except for visualization of acute hemorrhage or bone abnormalities.17,40,42 When referring a patient for MRI, clinicians should determine what area of focus will best demonstrate the suspected pathology (often the brain or orbit) and if gadolinium contrast or any special image processing techniques such as fat suppression, fluid attenuation inversion recovery (FLAIR) or diffusion-weighted imaging (DWI) should be included to maximize the likelihood of correct diagnosis (Figure 7).
Gadolinium contrast is used with T1W scans to enhance areas of a possible compromised blood-brain barrier, blood vessel abnormalities, inflammation and lesions.15,40,42,43 Contrast should be included for most neuro-ophthalmic conditions, baring a significant contraindication.17,43 Although gadolinium contrast is rarely contraindicated, the literature shows some reported cases of nephrogenic systemic fibrosis developing after use in patients with renal disease.30,43 Overall, the rate of adverse effects from the use of gadolinium is significantly less than that of the iodinated contrast medium used with CT.44
FLAIR is used with T2W imaging and when used, CSF will appear dark, whereas nearby areas of edema are bright.15,42 This allows demyelinating lesions to be more easily visualized.41-43
DWI is a sequence that can help reveal suspected acute ischemic changes—often within minutes—allowing for earlier detection compared with CT or conventional MRI.13,18,41-43
Fat suppression is used to suppress the hyperintense signal from substances such as fat and water, which can improve visualization of pathology and may be particularly useful for optic neuropathies and orbital lesions.43
 |
| Fig. 6. This figure shows normal MRI T1W images in (left to right) sagittal, coronal and axial cuts. Some major anatomic landmarks are indicated in red. Click image to enlarge. |
When to Order
Possible indications for MRI include unilateral or bilateral vision loss, efferent or afferent pupillary defects, diplopia, external ophthalmoplegia, lid abnormalities, ophthalmoscopic abnormalities indicative of intracranial disease and orbital trauma.45
Acute monocular vision loss. In these cases, pupil findings, appearance of the optic nerve, associated visual field involvement, patient symptoms and demographics may help narrow the diagnosis. Many causes of acute monocular vision loss can be diagnosed clinically or with additional lab or radiologic testing; however; if optic neuritis is suspected, MRI of the brain and orbit with gadolinium contrast, fat suppression (to show nerve enhancement) and FLAIR (which highlights demyelination lesions) would be indicated.13,17 Research shows that 95% of patients with optic neuritis demonstrate an enhanced optic nerve on MRI, and a single demyelinating brain lesion on MRI increases the risk for developing multiple sclerosis from 25% to 72% within 15 years.46,47
If progressive monocular vision loss is more chronic in duration, clinicians should suspect compressive, inflammatory or infiltrative lesions involving the optic nerve anterior to or at the optic chiasm.13,45 MRI of the orbit and brain with contrast and fat suppression would be suggested in this circumstance.13,17,45
Binocular vision loss. Particularly when presenting with visual field defects consistent with a suspected neurologic abnormality, this would typically indicate a lesion at or posterior to the optic chiasm.17
A bitemporal hemianopsia often presents as a result of a lesion that is causing chiasmal compression, most often a pituitary adenoma. For these cases, experts recommend MRI of the brain with contrast, and clinicians should communicate with the radiology department to ensure adequate focus on the chiasmal area (Figure 8).13,17,43 Urgent MRI or CT of the sella may also be indicated to rule out aneurysm or pituitary apoplexy, as they are important differential diagnoses when acute bitemporal field loss is detected.13,45
The appearance of a homonymous hemianopsia is typical of conditions involving the retrochiasmal visual pathway.13,43 In younger patients, MRI of the brain with contrast can help exclude a mass or demyelinating lesion as the suspected cause.13,17 Acute-onset homonymous hemianopsia in an elderly patient, however, is typically a result of an ischemic stroke; therefore, DWI should be included if symptoms are acute.17,45
Transient visual changes, early visual field disturbances (such as an enlarged blind spot) and headaches in the presence of bilateral disc edema are findings suggestive of increased intracranial pressure (ICP) and warrant expedient neuroimaging.6,17 An intracranial mass, meningitis, idiopathic intracranial hypertension (IIH) and cerebral venous sinus thrombosis are all among the possible etiologies.6 MRI of the brain with contrast would be indicated; however, magnetic resonance venography (MRV) may also be warranted when clinicians suspect venous sinus thrombosis.6,45
Mandatory neuroimaging must be completed prior to lumbar puncture in suspected cases of IIH.6,48 MRI findings typical of IIH include the appearance of an “empty sella” and a flattened appearance of the posterior globes.34
Horner’s syndrome. Ipsilateral ptosis, miosis and possibly anhydrosis are findings consistent with this condition, which can result from carotid dissection, apical lung tumor or any defect along the sympathetic innervation pathway to the eye.17 In the acute presence of head or neck pain, clinicians should rule out a carotid dissection.6,13,42,45 MRI of the head and neck with contrast and magnetic resonance angiography (MRA) or CTA of the head and neck is recommended in cases of Horner’s syndrome.6,13,45 Concern for an apical lung tumor (also known as Pancoast tumor) also warrants imaging of the chest, typically by CT with contrast.6,17
Ocular motility dysfunction. Clinicians should suspect a third nerve palsy with pupillary involvement is caused by a life-threatening aneurysm until proven otherwise.6,17,43 Emergent neuroimaging using contrast-enhanced CT/CTA or MRI/MRA is required.6,45 CT/CT angiography (CTA) are currently the techniques of choice due to better sensitivity and timeliness; however, MRI/MRA may be necessary should CT be contraindicated.6,43 Importantly, if initial neuroimaging does not reveal an aneurysmal cause and strong suspicion is still present, conventional catheter angiography should be considered.6,17,42
Isolated fourth or sixth cranial nerve palsies may or may not need imaging, since they are most commonly associated with an ischemic etiology.6,17,42 If a palsy presents acutely in a patient older than 50 who has vasculopathic risk factors (such as diabetes and hypertension), imaging may often be deferred as long as there are not accompanying neurologic signs or involvement, a history of cancer, and resolution occurs within 90 days.6,17,42 Otherwise, MRI of the brain with contrast would be indicated.
Non-cranial nerve ocular motility disorders such as skew deviation, nystagmus, supranuclear or internuclear ophthalmoplegia often also require imaging with MRI, typically with contrast and FLAIR.13,17,45 If acute transient ischemic attack or stroke is the suspected etiology, then DWI is also included.17
 |
| Fig. 7. This patient has thyroid eye disease, including proptosis OD>OS, secondary exposure keratopathy, extraocular muscle movement restrictions and diplopia. He has no clinical or radiologic evidence of optic nerve compression. Imaging includes (left to right, all axial cuts) CT with bone windows, standard CT, MRI T1W, MRI T2W and MRI FLAIR sequence. They reveal proptosis OD>OS, enlarged extraocular muscle bellies and mild prominence of the intraconal fat bilaterally. Click image to enlarge. |
When Not to Order
Although MRI may be the most clinically appropriate option, clinicians may forego ordering it due to absolute or relative contraindications. These include patients with magnetic metal in their body, such as cochlear implants and older generation aneurysm clips.17 A history of any metal in the body overall would at a minimum warrant a discussion with the radiology department to determine if MRI is advisable or if CT would be the preferred method of imaging.6,17
Cardiac pacemakers or implanted cardiac defibrillators are contraindications for MRI, since it can result in programming alterations and even failure.41
Claustrophobia is a relative contraindication, for which anxiolytic medications can help or, in some instances, open MRI testing can be substituted, although image quality may suffer as a result of poorer resolution.17 Significant obesity may also warrant the use of open MRI if the machine’s bore is too small for the patient.17
 |
| Fig. 8. This patient had a pituitary macroadenoma, which was resected via left pterional craniotomy. Preoperative (above) and postoperative (below) MRI T1W imaging shows (left to right) sagittal, coronal and axial cuts. Preoperatively, notice the pituitary fossa with a heterogeneously enhancing and enlarged pituitary extending to the left optic chiasm, which was displacing the left optic nerve’s course superiorly. Postoperatively, notice the hypo-enhancement in the sella turcica and removal of the suprasellar component of the pituitary lesion with minimal residual soft tissue and the decrease in the left optic nerve and chiasmal mass effect. The MRI images also show post-surgical encephalomalacia/gliosis in the opercular region of the left frontal lobe and in the anterior left temporal lobe, subadjacent to a left pterional craniotomy defect. Click image to enlarge. |
The decision to acquire diagnostic imaging may seem daunting for some clinicians, but it is crucial for the patient—and can be a life-saving decision for some. Clinicians who are familiar with the advantages, disadvantages and indications for the appropriate imaging modality won’t hesitate to order the right imaging when encountering these important clinical situations—and will reach a diagnosis in time to initiate appropriate treatment or refer to the correct care provider.
Drs. Fliegel, Seng, Weidmayer, and Lewis are staff optometrists in the VISN 10: VA Healthcare System–US Department of Veteran Affairs.
Contents in this publication do not represent the views of the US Department of Veterans Affairs or the United States Government.
1. Brant WE. Diagnostic imaging methods. In: Brant WE, Helms CE, eds. Fundamentals of Diagnostic Radiology. 3rd ed. New York: Lippincott; 2007:3-25. 2. Bushong SC. Essential concepts of radiologic science. In: Bushong SC, ed. Radiologic Science for Technologists Physics, Biology, and Protection. 10th Ed. Philadelphia: Elsevier; 2013:3-25. 3. Radiology Masterclass. Basics of x-ray physics. www.radiologymasterclass.co.uk/tutorials/physics/x-ray_physics_densities. Accessed May 23, 2018. 4. Lee CI, Elmore JG. Radiation-related risks of imaging. In: Aronson MD, Lee SI, eds. UpToDate. Waltham, MA. 5. Lawrence DA, Lipman AT, Gupta SK, Nacey NC. Undetected intraocular metallic foreign body causing hyphema in a patient undergoing MRI: a rare occurrence demonstrating the limitations of pre-MRI safety screening. Magnetic Resonance Imaging. 2015;33(3):358-61. 6. Bagheri N, Wajda BN, Calvo CM, et al. Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2017. 7. Williamson MR, Espinosa MC, Boutin RD, et al. Metallic foreign bodies in the orbits of patients undergoing MR imaging: Prevalence and value of radiography and CT before MR. Am J Roentgenology. 1994;162(4):981-6. 8. Kanal E, Brogstede JP, Barkovich AJ, et al. American College of Radiology White Paper on MR Safety. AJR Am J Roentgenol. 2002;178:1335-47. 9. Kaiser PK, Friedman NJ, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 4th ed. Philadelphia: Saunders; 2014. 10. West SG. Sarcoidosis. In: Hochberg MC, Gravallese EM, Silman AJ, et al., eds. Rheumatology. 7th ed. Philadelphia: Elsevier; 2019:175, 1470-79. 11. Braun J, Baraliakos X. Imaging of spondyloarthritis. In: Hochberg MC, Gravallese EM, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH eds. Rheumatology. 7th ed. Elsevier; 2019:125, 1024-32. 12. Tawfik HA, Abdelhalim A, Elkafrawy MH. Computed tomography of the orbit - A review and update. Saudi J Opthalmol. 2012;26:409-18. 13. Kim JD, Hashemi N, Gelman R, et al. Neuroimaging in opthalmology. Saudi J Opthalmol. 2012;26:401-7. 14. Shaw AS, Prokop M. Computed tomography. In: Adam A, Dixon A, Gillard J, et al. Grainger and Allison’s Diagnostic Radiology. 6th ed. Philadelphia: Elsevier; 2015:76-89. 15. Bowling B. Neuro-ophthalmology. In: Bowling B, ed. Kanski’s Clinical Opthalmology 8th ed. Philadelphia: Elsevier; 2017:773-849. 16. Gücük A, Üyetürk U. Usefulness of hounsfield unit and density in the assessment and treatment of urinary stones. World J Nephrol. 2014;3(4):282-6 17. Kruger JM, Lessell S, Cestari DM. Neuro-imaging: A review for the general ophthalmologist. Sem Ophthalmol. 2012:27:(5-6):192-6. 18. Bose S. Principles of imaging in neuro-opthalmology. In: Yanoff M, Duker JS. Opthalmology. 4th ed. Philadelphia: Elsevier; 2014:851-857.e1. 19. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37(2-4):1. 20. United States Nuclear Regulatory Commission. Backgrounder on biological effects of radiation. www.nrc.gov/reading-rm/doc-collections/fact-sheets/bio-effects-radiation.html. Accessed July 30, 2018. 21. American College of Radiology. Radiation dose to adults from common imaging examinations. www.acr.org/-/media/ACR/Files/Radiology-Safety/Radiation-Safety/Dose-Reference-Card.pdf?la=en. Accessed July 30, 2018. 22. Brenner DJ, Hall EJ. Computed tomography - an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-84. 23. Task Group on Control of Radiation Dose in Computed Tomography. Managing patient dose in computed tomography. A report of the International Commission on Radiological Protection. Ann ICRP. 2000;30(4):7. 24. Wakeford R, Little MP. Risk coefficients for childhood cancer after intrauterine irradiation: a review. Int J Radiat Biol. 2003;79(5):293. 25. Preston DL, Cullings H, Suyama A, et al. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008;100(6):428. 26. Yamazaki JN, Schull WJ. Perinatal loss and neurological abnormalities among children of the atomic bomb. Nagasaki and Hiroshima revisited, 1949 to 1989. JAMA. 1990;264(5):605. 27. Brent RL. Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol. 2009;200(1):4-24. 28. Kruskal JB. Diagnostic imaging procedures in pregnant and nursing women. In: Levine D, Barss VA, eds. UpToDate, Waltham, MA. 29. Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. 2009;39 Suppl 1:S4. 30. American College of Radiology ACR Committee on Drugs and Contrast Media. ACR manual on contrast media. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 30, 2018. 31. Davenport MS, Newhouse JH. Patient evaluation prior to oral or iodinated intravenous contrast for computed tomography. In: Bhalla S, Soto JA, Lee SI, eds. UpToDate, Waltham, MA. 32. Thomsen HS, Reimer P. Intravascular contrast media for radiography, MRI, and ultrasound. In: Adam A, Dixon A, Gillard J, et al. Grainger and Allison’s Diagnostic Radiology. 6th ed. Philadelphia: Elsevier; 2015;(2):26-51.e1. 33. Thust SC, Miszkiel K, Davagnanam I. Orbit. In: Adam A, Dixon A, Gillard J, et al. Grainger and Allison’s Diagnostic Radiology. 6th ed. Philadelphia: Elsevier; 2014:1556-89.e1. 34. Radgray.com. Hounsfield scale. www.radgray.com/ct/general-ct/hounsfield-scale. Accessed July 30, 2018. 35. Biousse V, Newman NJ. Neuro-ophthalmology Illustrated. 2nd ed.. New York: Thieme; 2015. 36. Costelloe CM, Chuang HH, Chasen BA, et al. Bone windows for distinguishing malignant from benign primary bone tumors on FDG PET/CT. J Cancer. 2013;4(7):524-30. 37. Shindler KS. Orbital disease in neuro-opthalmology. In: Liu GT, Volpe NJ, Galetta SL. Liu, Volpe, and Galetta’s Neuro-Opthalmology. 3rd ed. Philadelphia: Elsevier; 2019. 38. Kruger JM, Cestari DM, Cunnane MB. Systemic approaches for reviewing neuro-imaging scans in ophthalmology. Digit J Opthalmol. 2017;19;23(3):50-59. 39. Barkovich AJ, Raybaud C. Pediatric Neuroimaging. 5th ed. Philadelphia: Lippincott & Williams; 2005. 40. Johnson MC, Policeni BA, Lee AG, Smoker WRK, eds. Neuroimaging in Ophthalmology, 2nd ed. Oxford, NY: Oxford University Press; 2011. 41. Simha A, Irodi A, Davis S. Magnetic resonance imaging for the ophthalmologist: a primer. Indian J Ophthalmol. 2012;60(4):301-10. 42. Woods AD, Caputo MK. Essential uses of radiologic testing in eye care. Rev Optom. 2014;151(1):52-9. 43. Lee AG, Johnson MC, Policeni BA, Smoker WRK. Imaging for neuro-ophthalmic and orbital disease-a review. Clin Exper Ophthalmol. 2009;37:30-53. 44. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: Retrospective view of 456,930 doses. AJR App J Roentgenol. 2009;193(4):1124-7. 45. Lee AG, Brazis PW, Garrity JA, White M. Imaging for neuro-ophthalmic and orbital disease. Am J Opthalmol. 2004;138:852-62. 46. Rizzo JF, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2002;109(9):1679-84. 47. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727-32. 48. Gower DJ, Baker AL, Bell WO, Ball MR. Contraindications to lumbar puncture as defined by computed cranial tomography. J Neurol Neurosurg Psychiatry. 1987;50(8)1071-4. 49. MRI Technique. www.startradiology.com/the-basics/mri-technique. Accessed August 8, 2018. |
