Protocols and Pitfalls in Topical Steroid Use
It’s always a balancing act between benefit and side effects. Here’s how to keep patients safe while treating with steroids.
By Aaron Bronner, OD, and Walter O. Whitley, OD, MBA
Release Date: March 2, 2018
Expiration Date: March 2, 2021
Goal Statement: Topical corticosteroids can be a useful treatment strategy for everything from ocular surface disease to uveitis—but they can cause complications, and clinicians must account for their side effects. To better prepare clinicians to prescribe these agents safely, this article reviews the known side effects of ophthalmic corticosteroids, appropriate use and dosing-based differences among target tissues.
Faculty/Editorial Board: Aaron Bronner, OD, and Walter Whitley, OD, MBA
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 57001-PH. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: Dr. Bronner has no relationships to disclose. Dr. Whitley has a relationship with Alcon, Allergan, Bausch + Lomb, BioTissue, Carl Zeiss Meditec, Glaukos, TearScience, TearLab, Johnson & Johnson, OcuSoft and Beaver Visitec.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Iannucci and Francesca Crozier-Fitzgerald all have no relationships to disclose.
Topical corticosteroids are the most frequently prescribed class of ophthalmic agents by optometrists across the United States. In 2013, topical corticosteroids (including steroid/antibiotic combination agents) accounted for a little more than 50% of ophthalmic prescriptions written by ODs.1 Steroids were prescribed three times more frequently than topical antibiotics, topical antivirals and topical nonsteroidal anti-inflammatory drugs (NSAIDs) combined.1 And it’s no wonder we like these drugs, given their mechanism of action. Because essentially all nucleated cells in the body express receptors for these molecules, corticosteroids have a sweeping effect across many tissues.2,3
Regardless of dosing strategy (systemic or local), the therapeutic goal of corticosteroids is to limit the immune response via the phospholipid A2 pathway. Because of their wide range of target cells, corticosteroids have the broadest, but least specific, effect on inflammation of any immune modulatory agent.4
When dosed orally for systemic therapy, these agents reduce differentiation and maturation of immune cells within primary immune tissue, as well reduce the expression of pro-inflammatory cytokines and chemokines peripherally.4 When dosed topically, steroids don’t influence immune-cell maturation, but the effect on cytokines, chemokines and other pro-inflammatory molecules—an extremely robust mechanism of action—is preserved.4 By reducing the production of this legion of inflammatory mediators, locally dosed corticosteroids reduce several actions, including: vascular permeability to immune cells, the recruitment of other immune cells to that site, tissue breakdown, histamine release and subsequent edema, angiogenesis and fibroblast activation (which results in scar tissue), among other consequences.4
With these broad effects and their trickle-down clinical benefits, treating everything from ocular surface disease to uveitis, it’s easy to see why corticosteroids are such a favorite of the profession. However, although steroids are quite effective at addressing inflammation, they can cause complications. Common side effects of oral prednisone include increased intraocular pressure (IOP), cataract formation, mood changes and increased blood sugar levels. These are not associated directly with the desired mechanism of these agents and are truly unintended; with increased IOP possibly leading to irreparable vision loss via glaucoma, it’s particularly worrisome.
Another negative effect of steroid use is not an unexpected adverse event, but a predicable effect based on the mechanism at play. Because the primary effect of corticosteroids is limiting the immune response, their use may result in worsening of any condition where the immune response is needed (i.e., an infectious process). Unfortunately, although this effect can potentially lead to catastrophic vision loss, it is often under-recognized by the profession.
To better prepare clinicians to prescribe these agents safely, this article reviews the known side effects of ophthalmic corticosteroids, particularly within the cornea, their appropriate use and dosing-based differences among target tissues.
| This fulminant corneal ulcer was initially treated with Tobradex. Photo: Jeff Urness, OD |
Intraocular Pressure
Elevation in IOP is the most frequently observed side effect of ophthalmic steroid use, occurring in 18% to 36% of patients.5 Regardless of the specific steroid being used, this response is more likely to occur with prolonged dosing and has been observed in higher frequency in glaucoma suspects and patients.6 Research suggests the mechanism likely involves the alteration of the trabecular meshwork’s (TM) outflow efficacy via increased deposition of extracellular matrix within the TM.7 This is usually transient, though recalcitrant steroid-induced ocular hypertension is possible.
Ophthalmic steroid preparations come in varying strengths with equally varying risks of IOP elevation. For example, loteprednol 0.5%, rimexolone 1% and fluorometholone 0.1% are associated with a lower risk of steroid response, a lower peak pressure when a response occurs and a slower time to peak compared with dexamethasone phosphate 0.1% or prednisolone acetate 1%.8-10
The mechanism by which these steroids achieve their increased safety differs slightly between medications. In fluorometholone 0.1% and rimexolone 1%, liphophilicity plays a large part in reducing IOP spikes, as the medications don’t penetrate the cornea due to biphasic nature, thereby lowering the potential for spikes.11,12 Phosphate preparations, as hydrophilic solutions, penetrate the epithelium poorly, which may be ideal for ocular surface conditions.11,12
Hydrophobic alcohol-based and acetate suspensions, however, should theoretically be more adept at penetrating all layers of the cornea. Loteprednol achieves its enhanced safety, in part, from its reduced half-life in the anterior chamber as an ester-steroid, which reduces adverse drug reactions. The newest topical steroid, difluprednate 0.05%, seems to have the highest anti-inflammatory potency.13 This comes at a cost, however, as research shows the medication can cause the most extreme and most rapid IOP responses.14
Cataract Development
The literature shows significant variability in cataract formation in patients on systemic steroids, as between 6.5% and 38.7% of patients on them develop cataracts.15 Further research reveals varying associations with cataract and inhaled steroid compared with perinasal dosing.15 Whether topical, oral or inhaled, steroids are commonly associated with posterior subcapsular cataracts.15 Surprisingly, however, there is little to no peer-reviewed information regarding rates of cataract development with ophthalmic corticosteroids overall, let alone among specific agents.
Because of loteprednol’s soft drug design, research suggests it has a reduced potential to cause cataracts; however, this claim seems to be based on one retrospective review of 159 patients, none of whom developed cataracts while using loteprednol 0.2% for more than a year.16
Although the differences in the potential to cause cataracts among individual agents are still unknown, we do know that chronic topical steroid use, at least in cohorts with chronic intraocular inflammation, is associated with cataract formation—with greater dose frequency associated more frequently than lesser dosages.17 Practitioners can reasonably predict that long-term topical steroid use follows the same trends seen in IOP response among specific agents: those that don’t penetrate into the anterior chamber well and those that have a short half-life (fluorometholone, rimexolone and loteprednol) can be expected to produce cataracts at a lower rate than more potent steroids (prednisolone, dexamethasone and difluprednate).
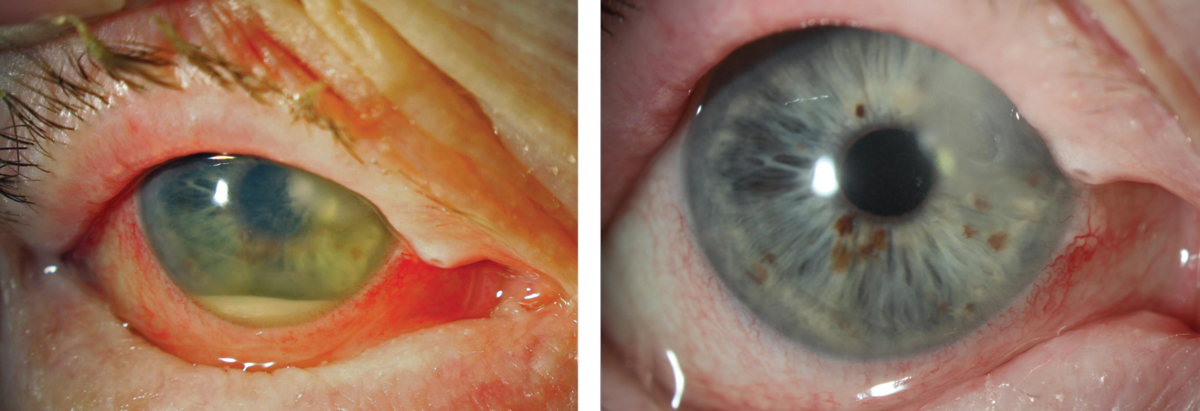  |
| When an ulcer requires steroids, they should be introduced cautiously. This Moraxella catarrhalis ulcer, at left, was treated for one week with medication that it was sensitive to (per culture results) and improved, but only slowly. It wasn’t until a steroid was cautiously introduced that the eye totally quieted, at right. In this case, having a culture result in hand to ensure appropriate antimicrobial treatment made the subsequent addition of a steroid a reasonable step to take. Click images to enlarge. |
Selection and Dosing
When dosing corticosteroids for inflammation of the ocular surface, select a poorly penetrating agent (fluorometholone or rimexolone) or soft drug corticosteroid such as loteprednol to reduce risk of complications.
When inflammation is in the anterior chamber or deeper cornea, the selected agent should balance with the degree of anti-inflammatory effect necessary. For mild to moderate inflammation, loteprednol is a good option, and more severe cases may do well with prednisolone acetate 1%. In particularly severe cases of corneal or anterior chamber inflammation, the only topical medication we’ve had success with is difluprednate; otherwise, you’re on to sub-Tenon’s dosing or systemics.
Excess steroid use can be nearly as harmful as chronic inflammation and is of particular concern with long-term management of inflammation as seen in chronic keratitis (as seen occasionally with herpes zoster ophthalmicus), prevention of corneal graft rejection and cases of chronic uveitis. When deep penetration is unnecessary but chronic therapy is expected, a more superficially acting steroid is appropriate, and in all cases the lowest dose that fully controls inflammation should be used.
With more substantial steroids and the increased potential for steroid complications over an abbreviated time frame, the follow-up interval should be appropriately reduced.
Immune Response and Tissue Function
Beyond the capacity for creating IOP spikes and cataracts, steroid use comes with tissue-specific precautions. Optometrists typically prescribe corticosteroids for three primary anatomic zones: the ocular surface (including lids, conjunctiva, episclera and corneal epithelium), the non-superficial corneal layers and the anterior chamber. Steroid use for each of these zones carries a risk for the development of cataracts and glaucoma, depending on the specific agent and duration of treatment. The risks also differ based on the consequences of potentiating infection.
Conjunctiva. True conjunctival infections, with the exception of hyper-acute conjunctivitis, can generally be treated with corticosteroids with near impunity and without fearing induction of vision-threatening escalation of the disease. The reason for this has to do with the tissue function, its role in vision and the normal immune response.
A mucous membrane, the conjunctiva is essentially a watershed between the external and internal environments. As with all mucous membranes, one of the conjunctiva’s primary functions is to act as a barrier. The most effective barrier tissue of the body is the skin. Non-nucleated, keratinized tissue, which makes up the most external layer of the skin, creates a robust physical barrier to microbes and is able to repel most infections. In fact, it is nearly impossible for a virus to infect healthy, uncompromised skin.18
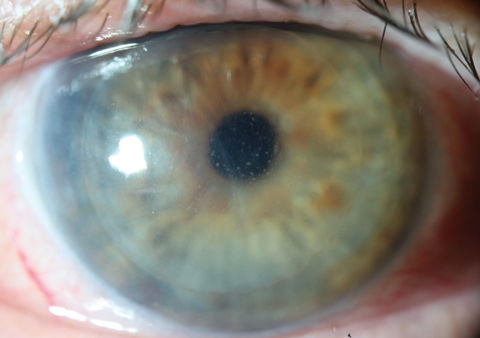 |
| A rejection of a DSAEK graft manifesting as corneal edema and scattered keratic precipitates. This appearance also approximates that seen with viral endotheliitis; in both cases, topical steroids are indicated. Click image to enlarge. |
As a mucous membrane without the benefit of keratinized tissue, the conjunctiva is at a disadvantage relative to the skin at preventing infection; therefore, infectious conjunctivitis is more likely than infectious dermatitis. These conjunctival infections, however, are almost always self-contained and have minimal long-term consequences because what the conjunctiva lacks in keratinized tissue it makes up for with immediate access to the immune response. The conjunctiva is host to secondary lymphoid tissue (conjunctiva-associated lymphoid tissue), has a rich vascular supply and, further, is not directly responsible for visual function—allowing some tissue damage without impact on function.19
Due to the conjunctiva’s immediate access to the immune response, topical steroid use may reduce the degree of the conjunctival immune response without eliminating it entirely. Steroids can be a useful adjunct in the therapeutic and palliative management of both inflammatory and infectious conjunctivitis by reducing unnecessary inflammation that can lead to pain, conjunctival fibrosis, destruction of goblet cells and symblepharon formation.
Clinicians must remember that infectious conjunctivitis should be preferentially treated with anti-infective medications. Still, it may be difficult to differentiate infectious from non-infectious, and given the access to local immune response, treatment of a localized infectious conjunctivitis with steroid will not lead to profound tissue destruction, unlike that which may occur within the cornea. Thus, combination drops (paired antibiotic/corticosteroid) are safe and effective in most cases.
Likewise, anterior uveitis stemming from disseminated systemic infections such as tuberculosis, syphilis, Lyme disease and leprosy may be treated with topical corticosteroids without risk of exacerbating the underlying disease process. Of course, these patients all need systemic anti-infectives to cure their disease, but topical steroids are safe, may enhance treatment by reducing inflammatory sequele and may reveal an infectious etiology if you see an incomplete clinical response to anti-inflammatory treatment.
Although the uveitis generated by these etiologies often exhibits an incomplete response to topical corticosteroids, topical steroid use is always appropriate front-line therapy for cases of anterior uveitis.20
The same cannot be said for systemic steroid use for cases of infectious uveitis, however. Though systemic corticosteroid agents may be indicated on a case-by-case basis for infectious disease management (shingles treatment is often supplemented with oral prednisone, and some severe leprosy-related immune reactions necessitate their use), they should not be used for all cases. They have been known to exacerbate the underlying disease and possibly even lead to death.21
When using topical agents to control a severe uveitis, clinicians should not shy away from strong initial dosing. Anecdotally, treating uncontrolled anterior uveitis with prednisolone acetate 1% QID often meets with predictably minimal success. In cases of moderate to severe acute anterior uveitis, clinicians should prescribe frequent, often hourly, dosing of a strong steroid such as difluprednate, which has been shown to be non-inferior to prednisolone acetate for anterior uveitis when dosed half as frequently, and only taper gradually once inflammation is totally controlled.22
Cornea. Corticosteroids are often necessary to facilitate a good, prompt resolution of corneal pathology; however, when used incorrectly they may result in catastrophic vision loss. The specific implications and risks associated with steroid use in the cornea revolve around the cornea’s function and immune response.
The cornea has two primary functions: an optical interface that transmits and refracts light and a barrier to the external environment. However, maintenance of one sets up challenges for the other. The external cornea is part of the ocular surface mucous membrane; however, unlike most other mucous membrane-derived tissue, the cornea has no local lymphoid tissue, few native immune cells and no local vascular supply. Presence of any of these at high levels would enhance corneal barrier function through increasing access to the immune response, but would result in a loss of corneal clarity and reduced optical function. However, the absence of these features from the central cornea limits the cornea’s ability to function as a barrier. If the cornea develops an infection, the immune system is initially unable to help, which allows for early unfettered proliferation of the microbe.
Once an immune response is mounted, corneal optical function is sacrificed to preserve the barrier function, as resultant inflammation often contains the infection at the expense of a corneal scar. Because of the inherent antagonism of the cornea’s barrier and optical functions, which are precariously balanced in a healthy eye and are thrown out of balance in an infected cornea, the use of corticosteroid in infected corneas has significant possible ramifications.
| Corneal Transplants Though not all of the blame falls to steroid use (some mechanical strain on the TM occurs during the surgical procedure itself), steroid-induced glaucoma is responsible for a large percentage of cases.1 Likewise, microbial keratitis is up to 55 times more likely in patients with a PK compared with normal extended wear soft contact lens users.2,3 These eyes are also much more likely to have poor outcomes with medical management. The rate of re-graft as a result of infection is as high as 57%, and only 14% achieve 20/200 or better vision with medical management.2,4 Though infection in a PK population cannot solely be pinned on the use of corticosteroid, given the wide variety of compromises that occur to the ocular surface with PK, steroid use is certainly among the factors leading to a greater rate of infection and a worse prognosis. Fortunately, with lamellar grafting, these risks diminish, either due to reduced necessity of steroid, as with deep anterior lamellar keratoplasty (DALK), or reduced disruption on immune privilege as with DSAEK and DMEK. However, a certain patient population continues to need PK, and though risk may be reduced with lamellar surgeries relative to PK, these eyes still need steroid use for between one and two years depending on the transplant type. They also need observation to catch any complications of the graft or the treatment. For average risk of transplants in steroid-responding PK patients, research shows loteprednol is less likely to result in IOP spiking and does not increase risk of rejection.5 Likewise, in lamellar surgery, loteprednol seems to effectively prevent rejection while reducing IOP issues.6 Fluorometholone 0.1%, while also effective at controlling IOP issues compared with prednisolone, was associated (somewhat predictably given its poor penetrance into the deep cornea) with a higher rate of possible rejection episodes.6,7
|
A now somewhat frequently encountered misconception is that the Steroid for Corneal Ulcers Trial (SCUT) shows no benefit or harm in the adjunctive use of corticosteroids for corneal ulcers; therefore, some think we can use steroids for corneal pathology with impunity.23 This is a dangerous clinical mindset and a misinterpretation of the SCUT study and its findings. The study does not show there was no benefit or harm in the use of corticosteroids for corneal ulcers. It shows, instead, no benefit or harm with light use of corticosteroid (prednisolone phosphate 1% QID for one week, BID for one week, then QD for one week) in the management of bacterial keratitis when attempts were made to sterilize the cornea prior to institution of steroid.23 All patients in this study were placed on moxifloxacin hourly for two days prior to being grouped into steroid plus antibiotic group or antibiotic plus placebo groups.23 Therefore, conservative steroid use as an adjunct therapy only follows SCUT protocol when attempts to sterilize the cornea are made prior to initiation of steroid use—a critical distinction.23
Due to the lack of immune response held by the central cornea, further suppression of it with corticosteroids prior to ensuring an effective antimicrobial sterilization (as assessed with a positive response to therapy) may result in spread of the ulcer and treatment failure. In addition, while the SCUT study was inconclusive on conservative use of corticosteroid in bacterial keratitis following an attempt at sterilization, we know from other retrospective reviews that steroid use with corneal ulcers increases the likelihood of progression towards outcomes requiring surgical management. More than 50% of patients from one series on infectious keratitis that went on to require keratoplasty had been on previous steroid (compared with 18% of the ulcers that did not require surgery).24 Likewise, nearly 25% of patients in another study on eyes requiring enucleation or evisceration for infectious keratitis had been on topical corticosteroids.25 These outcomes are critical to guard against; thus, the cavalier use of corticosteroids must be avoided when dealing with corneal pathology.
While most eye care providers know to avoid corticosteroid use with infectious keratitis except in an extremely conservative manner, the same is not always true with combination therapies, often prescribed for “mystery keratitides.” These have the most potential to do harm because of the flawed assumption that the antibiotic agent in the combo drop covers against infection while the anti-inflammatory covers for inflammation. In the central and paracentral cornea, the absent native immunity may not allow low-dose antibiotics to cover for the deleterious effects of the steroid when faced with an infectious challenge. Tissue concentrations of the antibiotic in these agents may be insufficient to slow colonization of the offending microbe, and the immune suppressing effect of the steroid may allow further spread by reducing immune cell migration and clearance of the infection. These possibilities are particularly concerning given that, in this setting, any worsening of infection may lead to a permanent worsening in vision.
It is crucial to know the corneal pathology you are dealing with prior to initiating combination or steroid therapy for any keratitis. Anecdotally, a good rule of thumb is to avoid using a steroid for any isolated ulcerative process of the central and paracentral cornea. If you have an infiltrate and an epithelial defect, it’s likely you are dealing with an infectious process.
The possible exception to this rule is a lesion in the far corneal periphery, as these are typically hypersensitivity/immune-mediated reactions, which will respond well to combination drops. In the rare event a far peripheral ulcer is infectious—which is uncommon near the limbus—proximity to the immune response will generally prevent dramatic escalation until initiating more appropriate therapy. Additionally, scarring in the far periphery is much less likely to cause reduced vision.
Though we aren’t promoting cavalier use of steroid on peripheral corneal pathology, the immunology and function of the peripheral cornea allows for more forgiveness in outcomes, should your treatment be incorrect. As with all corneal pathology, follow-up should be short when introducing a steroid to identify any worsening. For non-peripheral keratitis, stromal inflammation that is small, non-ulcerated and multifocal (i.e., contact lens-associated red eye, Thygeson’s and adenoviral infection) generally responds robustly and safely to corticosteroid.
Though inappropriate use of steroid on bacterial, protozoan or fungal keratitis may lead to poor visual outcomes, exacerbation of viral keratitis is probably the most frequently described negative corneal sequela of corticosteroid use in optometry. It is certainly true that treating herpes simplex virus (HSV) infections (clinically, this refers to the dendritic spectrum of disease, as well as the rare HSV-derived necrotizing stromal keratitis) with a steroid will worsen the infectious episode and increase superficial scarring.
The pathognomonic finding of a dendrite is a telltale sign that a steroid is inappropriate; however, it can be more challenging to differentiate dendritic lesions near the limbus from hypersensitivity reactions, often leading to the somewhat tongue-in-cheek “steroid provocative test.”
For other forms of herpetic keratitis, such as stromal keratitis and endotheliitis, topical steroids are an important part of the therapy, though their use should generally be paired with an antiviral to reduce the risk of reactivation.
For any significant unilateral vascularization, especially with a nonulcerated stromal keratitis, you are probably dealing with an immune-mediated process, likely HSV immune stromal keratitis, and may safely pair a corticosteroid with oral or topical antivirals. The same treatment holds in cases of unilateral acute corneal edema without stromal infiltration or dramatically elevated IOP, which is generally HSV endotheliitis. A sight-threatening infection of the cornea resulting in this clinical constellation is rare.
Laterality is also helpful in distinguishing what keratitides are “steroid safe.” Though it is possible that bilateral corneal inflammation may be infectious in origin, it’s unlikely—approximately 97% of HSV keratitis is unilateral and, barring exceptional risk factors such as bilateral corneal surgery, nearly all cases of microbial keratitis are unilateral.26 Although the same rules of avoidance hold—infiltrated ulcerations of the mid-periphery, paracentral or central cornea should avoid steroid—most other bilateral keratitides are safely treated with a steroid. In our experience, the most common sources of bilateral corneal inflammation are Staphylococcal hypersensitivities, rosacea keratitis, Thygeson’s, contact lens reactions and postviral epidemic keratoconjunctivitis, all of which respond well to corticosteroid or combination drops.
Because inappropriate steroid use on the cornea can have a devastating impact, follow up should be reduced when adding a steroid to evaluate for a negative response. All patients also should be counseled to call the office if the condition worsens.
Without a doubt, corticosteroids have earned their place among the class of ophthalmic agents most frequently prescribed by optometrists. Their ability to aid in the management of almost all forms of ocular inflammation, even those inflammations derived from infectious processes, means their indications for use are without peer. This familiarity, however, can breed complacency, which can lead to vision loss. For all ophthalmic steroid use, monitoring chronic use for adverse effects, particularly increased IOP, is critical. And when prescribing them, be sure to anticipate possible tissue-specific complications and adjust your follow-up schedule appropriately.
Dr. Bronner is an attending optometrist at Pacific Cataract and Laser Institute in Kennewick, WA.
Dr Whitley is the director of optometric services at Virginia Eye Consultants in Norfolk, VA.
1. Gonzalez A, Lakhani R, Bennett N, De Paz C. A twelve quarter quantitative analysis of ophthalmic drugs prescription writing by optometrists in the United States. Clinical Optometry. 2014;6:5-10. |
