Overcoming Secondary Glaucomas
A proper understanding of these conditions is paramount in providing swift and appropriate care.
By Michael Cymbor, OD, and Jenae Stiles, OD
 |
Release Date: November 15, 2019
Expiration Date: November 15, 2022
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe the fundamental differences between primary and secondary glaucoma.
- Identify the presentation of various forms of secondary glaucomas.
- Perform the necessary elements of the patient history, ocular examination/vision testing as well as diagnostic testing and/or imaging, and any other ocular or systemic evaluation required to diagnose secondary glaucomas.
- Provide, or otherwise obtain, the ocular and systemic treatment that the patient requires.
Target Audience: This activity is intended for optometrists engaged in the care of patients with secondary glaucomas.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Michael Cymbor, OD, and Jenae Stiles, OD.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 65238-GL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Stiles has nothing to disclose.
Dr. Cymbor has fees from non-CME/CE services from Optovue.Dr. Ding has nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
In optometry school, we were taught that glaucoma converts and progresses over years and years. “Monitor” is the mantra. But sometimes, glaucoma isn’t a slow and steady disease.Secondary glaucoma refers to any form of glaucoma in which there is an identifiable cause. The primary purpose of this article is to highlight the aggressive nature of two secondary glaucomas—exfoliation glaucoma (XFG) and, to a lesser extent, pigmentary glaucoma (PG)—as their aggressiveness is often under-appreciated. Other secondary glaucomas—including uveitic, traumatic and lens-induced are also discussed.
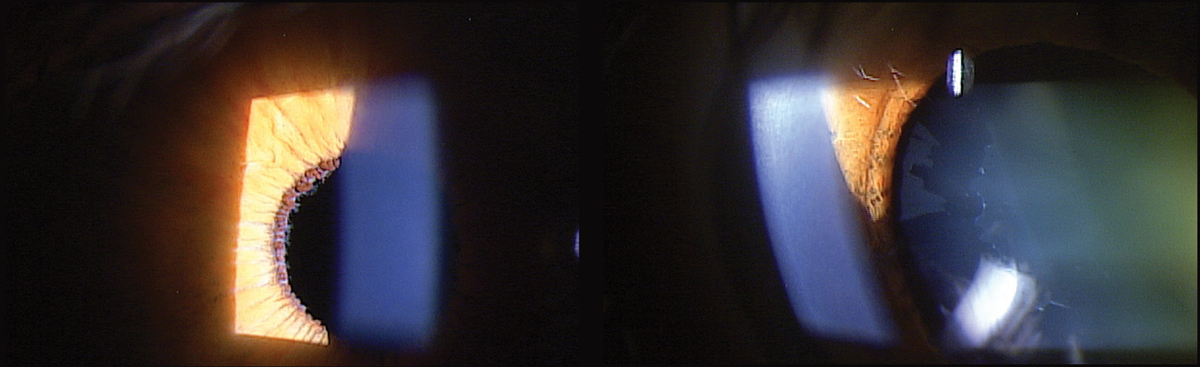 |
| Fig. 1. At left, XFS material deposited on the pupillary margin of a 71-year-old patient diagnosed with XFS. At right, note the classic XFS pattern deposited on the anterior lens capsule. Click image to enlarge. |
Exfoliation Syndrome
Exfoliation syndrome (XFS) may cause an extremely aggressive form of glaucoma. The severity of exfoliative glaucoma (XFG) may be overlooked due to the relatively slower progression of primary open-angle glaucoma (POAG). With failure to diagnose/delayed diagnosis being the most common reason for malpractice among all physicians, it is imperative that a timely diagnosis be made to tailor treatment.1
XFS was first described in 1917 and was termed pseudoexfoliation in 1953 when pseudocapsular deposits were found on histological sections of three eyes with clinical exfoliation.2,3 The term exfoliation described changes to the lens from capsular delamination due to high amounts of infrared radiation, commonly found on the natural lenses of unprotected steel foundry workers.
A return to the original nomenclature coined in 1917 is now occurring. Due to the current rarity of true exfoliation, the term pseudoexfoliation is being replaced by exfoliation. Both terms are currently found in the literature. For simplicity, we will use XFS and XFG in this article.
XFS affects 70 million people worldwide, with an emphasis on those of European descent.4 At one time, XFS was considered solely a Scandinavian disease, but now prevalence patterns vary greatly. Studies report that it affects approximately 20% to 25% of those older than age 60 in Iceland and Finland, with virtually no signs of XFS found among the Eskimo population and very low prevalence reported in the Japanese.5
| Patient with XFS Nearly Lost to Follow Up A 71-year-old white female presented to the office in 2013 for a comprehensive eye examination. She complained of changes in her distance vision. Her IOP by non-contact tonometry (NCT) was 17mm Hg OD and 15mm Hg OS. On exam, the optometrist noted XFS material deposited on the left pupillary margin along with a classic bull’s eye pattern on the left lens on dilated fundus exam (Figure 1). The cup-to-disc ratio was noted to be 0.4/0.5 OD and OS. Optical coherence tomography (OCT) was performed the same day, which showed a thick retinal nerve fiber layer (RNFL) OU with a relatively thick ganglion cell complex (GCC) (Figure 2). The patient was diagnosed with exfoliation syndrome OS and educated on the serious nature of the diagnosis. She was scheduled for a complete glaucoma work-up in one month. The patient did not show for the follow-up visit and was not seen again until 2015. That exam again showed the bull’s eye capsular pattern OS with IOPs of 19mm Hg OD and 18mm Hg OS by NCT. The cup-to-disc ratio was again graded at 0.4/0.5 OD and OS. The patient was again educated on the serious nature of the diagnosis and was scheduled for a glaucoma work-up in two weeks. Again, the patient never showed, but a concerted effort (which included phone calls, letters and a certified letter) was made to encourage the patient to return to the office. Unfortunately, the patient did not return again until 2017. At this visit in 2017, IOPs measured 15mm Hg OD and OS by NCT. OCT revealed significant RNFL and GCC thinning OS, and her cup-to-disc ratio was now graded at 0.5/0.65 OD and OS (Figure 3). She was diagnosed with exfoliation glaucoma OS. She was also diagnosed with grade 2 nuclear sclerotic cataracts OU. The optometrist started the patient on one drop of Lumigan (bimatoprost, Allergan) every night OU and referred her to the Glaucoma Institute of State College. Glaucomatous visual field testing showed a small depression OD and a significant reduction OS (Figure 4). Treated pre-dilation IOPs measured 10.6mm Hg OD and 17.1mm Hg OS by Ocular Response Analyzer (ORA). Post-dilation IOPs measured 12.1mm Hg OD and 31.3mm Hg OS by ORA. At this time, she is scheduled for cataract surgery and Kahook Dual Blade goniotomy OU. |
XFS is characterized by fibrillar deposits in virtually all tissues within the anterior segment and some tissues within the posterior segment. These deposits are caused by an imbalance of enzymes called matrix metalloproteinases (MMPs).8 This systemic extracellular matrix disorder is thought to be triggered by both genetic and environmental factors, causing irregular cellular degeneration, abnormal lysosomes and mitochondria and disorganized microtubules essential for maintaining cellular integrity.9
While several genes are implicated in XFS, the strongest association is linked to the lysyl-oxidase-like 1 (LOXL1) gene.10 LOXL1 controls a key enzyme in extracellular matrix formation. This gene is essential for the covalent crosslinking of collagen and elastin in connective tissue.11 LOXL1 expression and XFS pathogenesis can be influenced by UVB exposure, oxidative stress and hypoxia, among several other environmental factors.12 Higher latitude, high caffeine intake and low dietary folate consumption are also associated with increased risk of XFS.13
Approximately 30% to 50% of patients with XFS develop glaucoma, and this aggressive ocular disease is the most common identifiable form of secondary open-angle glaucoma, mostly affecting those in the seventh and eighth decade of life.14 XFG accounts for 20% of all glaucoma cases worldwide and is also associated with pathology of several other ocular structures.
Because of the relatively high risk of XFS converting to XFG, we recommend following all XFS patients at six-month intervals.
Ocular Targets
The more commonly recognized ocular targets include the lens capsule, lens zonules, iris and the angle.
XFS weakens the lens capsule, resulting in the classic bull’s eye pattern on the lens, which is caused by the mechanical rubbing against the pupillary frill, and best visualized upon dilation.15 A careful inspection of the pupillary frill will reveal whitish fibers that can be visualized even if the patient is pseudophakic. This mechanical friction releases iris pigment with resultant iris transillumination defects that occur closer to the pupillary margin, in contrast to pigmentary glaucoma in which transillumination defects occur in the mid-periphery.
XFS fibers can be found on the zonules, which can be visualized in a fully-dilated pupil or during dilated gonioscopy. XFS fibers on the zonules usually indicate zonular weakness. Direct signs of zonular weakness include subluxation of the lens, zonular dialysis and phacodonesis.16 A shallow anterior chamber depth, due to the forward shift of the lens, can be an indirect clue of zonular weakness.17 Zonular weakness may cause the lens to move either anteriorly or posteriorly, causing refractive asymmetry. Pupil asymmetry can also be indirect sign of this complication.
Zonular weakness can present challenges during cataract surgery, especially with initiation of the capsulorhexis, as too much zonular tugging may cause the fragile zonules to break.18 It is important to communicate the presence of exfoliation to the surgeon during the referral to ensure they take extra surgical caution.
In addition to fiber buildup on the pupillary frill, the iris may have endothelial cell loss that may include damage to the dilator muscle. This can result in asymmetric pupil dilation during the comprehensive eye exam. Iris angiography in XFS patients has shown nonperfusion and, as mentioned, transillumination defects at the pupillary margin are common.19
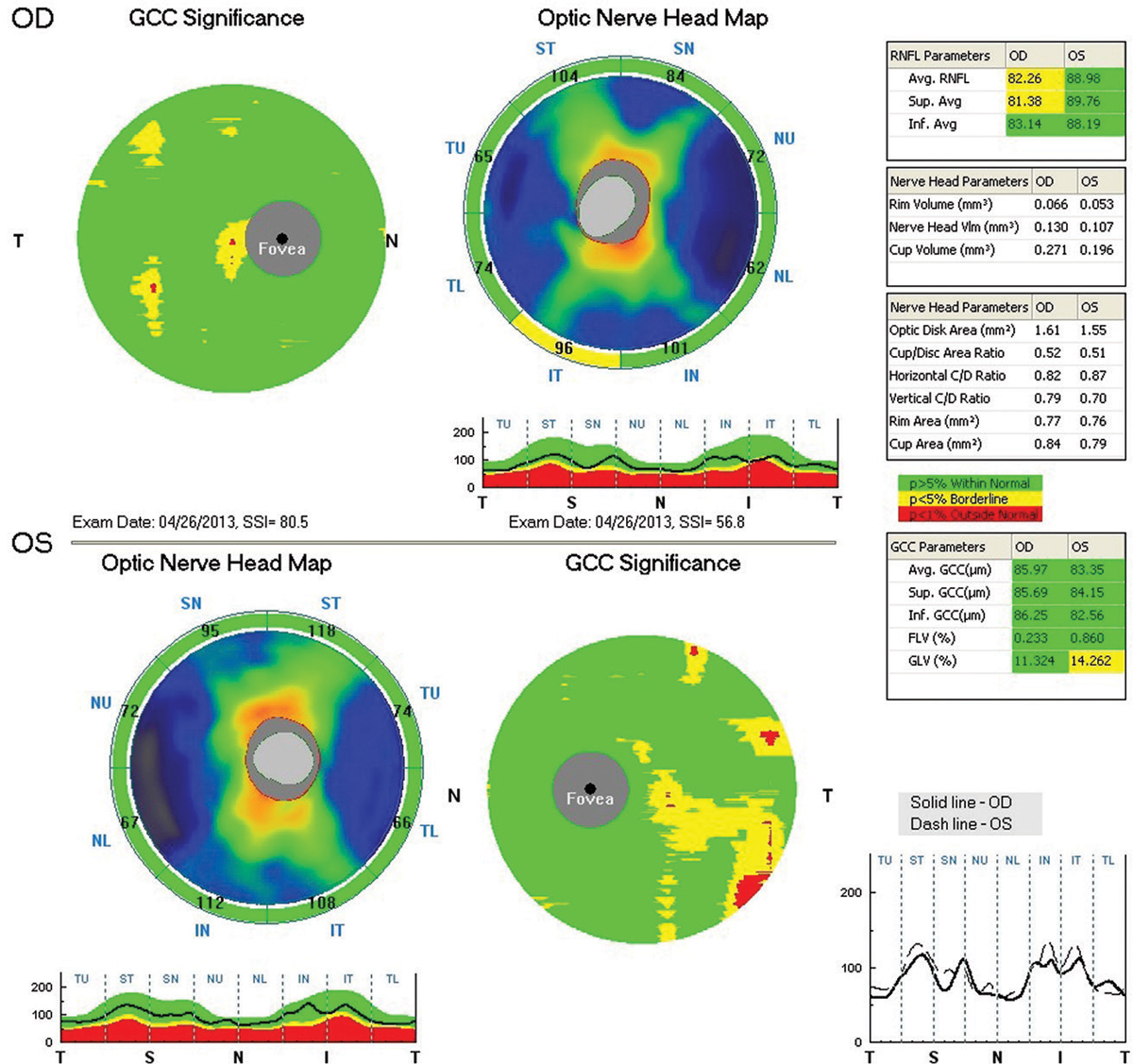 |
| Fig. 2. RNFL and GCC OCT imaging at presentation in 2013 shows a thick RNFL OU and relatively thick GCC OU. Click image to enlarge. |
The angle will show increased pigment in the anterior trabecular meshwork that is more irregular than pigment dispersion syndrome. The trabecular meshwork pigmentation has a “brown sugar” appearance. Pigment on Schwalbe’s line may occur and is known as Sampaolesi’s line. XFS fibers can clog the angle and arise both from the iris-lens capsule interaction as well as angle cells themselves.20 Eventually, the trabecular meshwork and Schlemm’s canal become disorganized, leading to abnormal function. Zonular weakness can also lead to a narrow angle due to forward displacement of the lens.21
There are several ocular targets for exfoliation material—including the lamina cribrosa, corneal endothelium and ciliary body—that are less commonly recognized because they are more subtle or more difficult to analyze.
The lamina cribrosa is the sieve-like, load-bearing connective tissue that separates two very different pressure environments, namely the intraocular pressure (IOP) and the cerebrospinal fluid pressure. As the LOXL1 gene is responsible for connective tissue maintenance, XFG displays a thinner lamina cribrosa compared with POAG eyes.22 This weakened integrity may crimp axons and blood vessels running through it, contributing to the more rapid progression of the disease.
XFS deposits and pigment cells build up on the corneal endothelium, causing stress to these cells and early cell death.23 There is an increased risk of corneal decompensation, especially with IOP fluctuations from a shallow anterior angle. Pre- and postoperative cataract surgical care should include endothelial cell counts. XFS fibers may build up on the ciliary body, giving it the appearance of newly fallen snow. This can lead to the aberrant insertion of the zonules into this muscular structure, contributing to zonular weakness.23
Exfoliation may be apparent in many, if not most, of the ocular structures mentioned above but may not necessarily be found in all.
Systemic Targets
As experts of the eye, optometrists must consider not only the ocular effects of XFS/XFG, but also the systemic complications that are common with this syndrome. In reality, the ocular changes we see are the manifestation of a systemic disease, and we must remember that the clinical course of XFS/XFG may involve alerting the primary care physician of its existence and any systemic correlations. It may also involve referrals to other specialists for complete care.
XFS fibers have been found in the heart and in the small blood vessels supplying the heart.24 The Blue Mountains Eye Study suggested that a combined history of angina, myocardial infarction and stroke are significantly associated with the presence of XFS.25 Myocardial ischemia and aortic aneurysms are also associated with XFS and XFG.24 This material can also deposit in the lungs. Due to the age of these patients at presentation, there is a significant probability that a cardiologist has already been involved in their care, but consider a referral to this specialty if care has not been established.
When considering any pathologic process affecting the small blood vessels, do not overlook the kidneys. A significant association with XFS and renal artery stenosis exists.26
While affecting the small blood vessels in the brain, XFS fibers can also deposit on the meninges.27 Several studies show a higher frequency of Alzheimer-related dementia in patients with XFS.28 The severity and prevalence of sensorineural hearing loss in patients with XFS is increased, lending the need to consider audiologic testing to improve the quality of life of these patients.29
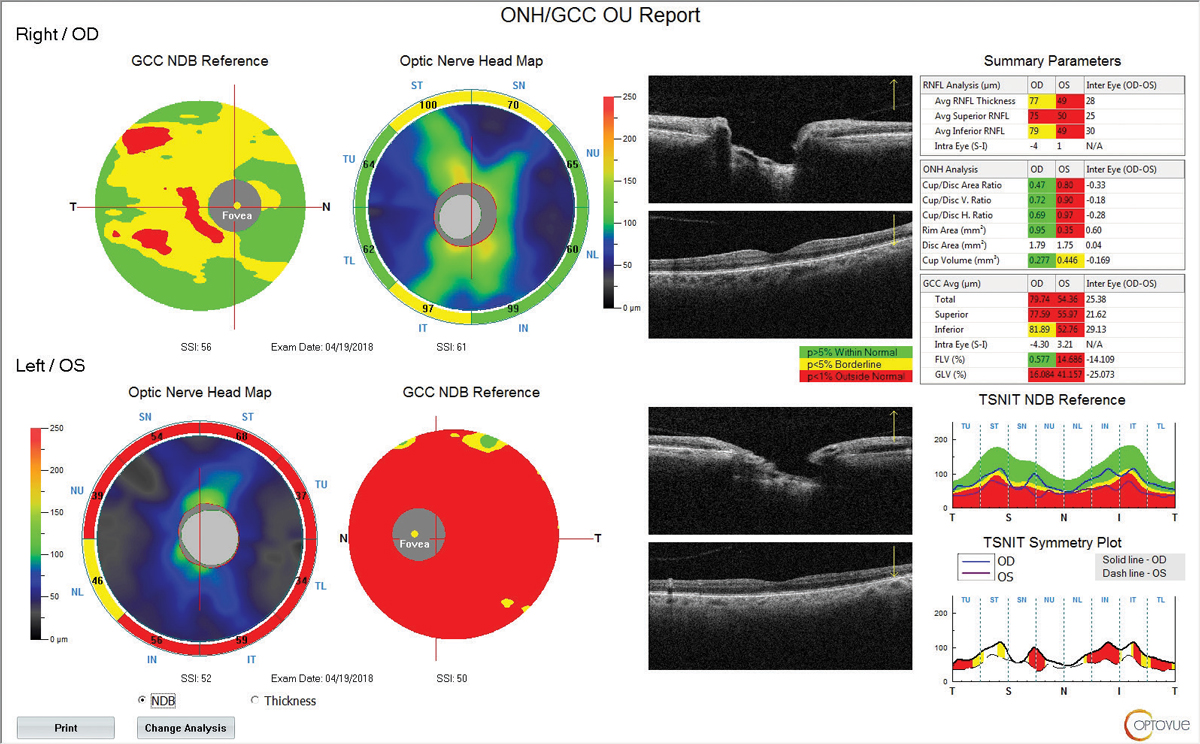 |
| Fig. 3. RNFL and GCC OCT at follow up in 2017 shows significant RNFL and GCC thinning of the left eye. Click image to enlarge. |
Current Treatment Strategies
Prostaglandins are effective first-line therapy for treating XFG. Pilocarpine 2% at night can successfully reduce lens-iris interaction (which reduces exfoliation fiber migration to the trabecular meshwork), but it can cause posterior synechiae and exacerbate preexisting anterior subluxation of the lens due to zonular weakness.21 A 2009 study showed that latanoprost and pilocarpine 2% was more effective than aqueous suppressants. Researchers have speculated that aqueous suppressants may actually allow more fibers to accumulate in the trabecular meshwork, leading to worsening of function over time.30 Further study is needed in this area.
If medical therapy fails or a patient is not compliant, consider surgical options. Selective laser trabeculoplasty (SLT) and argon laser trabeculoplasty (ALT) are both effective treatments for XFG.31 Patients with XFG tend to experience more fluctuation of IOP and require a greater reduction of mean 24-hour IOP than those with POAG, leading to initiation of combination therapy much sooner.32 SLT/ALT causes more inflammation in XFG patients than in POAG patients, so those with XFG must be monitored more closely for IOP spikes in the postoperative period. Overall, XFG patients must be monitored more frequently than POAG patients, with consideration of surgical options sooner.
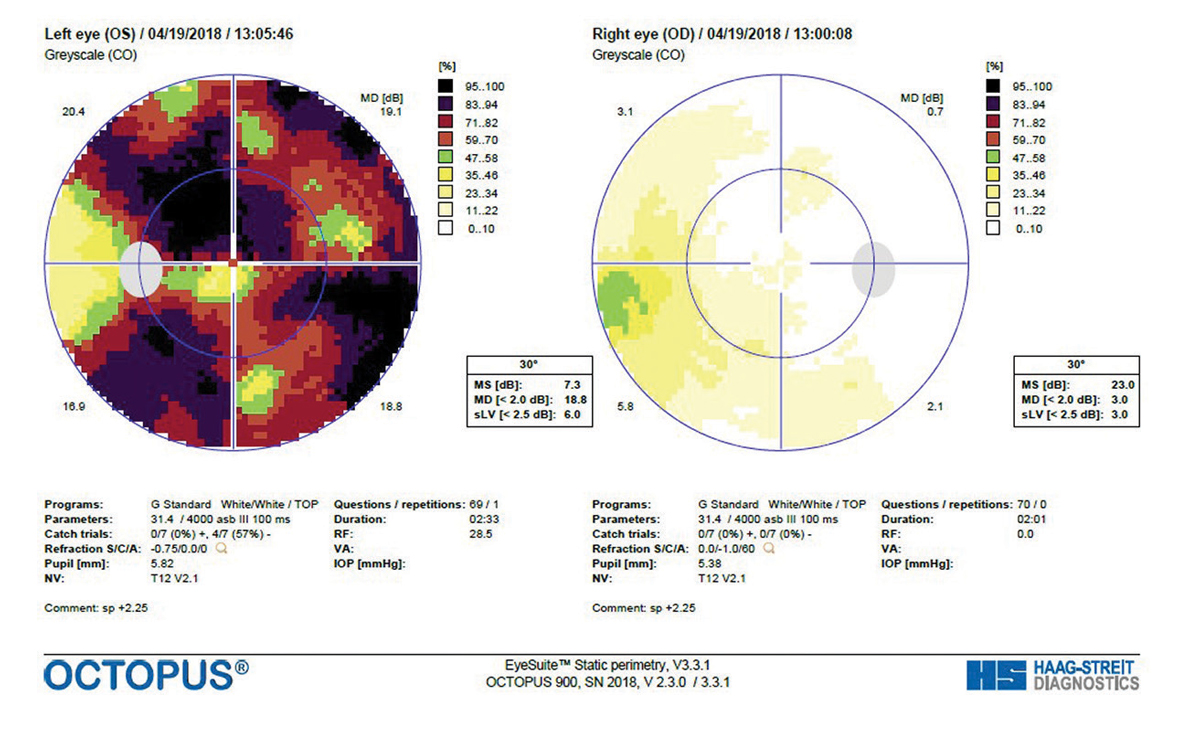 |
| Fig. 4. A GTOP visual field completed in 2017 shows a small depression of the right eye and significant reduction of the left eye. Click image to enlarge. |
Cataract surgery must also be approached with more caution in XFG patients. While cataract removal may reduce XFS fibers in the trabecular meshwork, weak zonules may pose several potential complications for the surgeon and result in a smaller capsulorhexis. This smaller opening can lead to an increased risk of phimosis (i.e., the centripetal fibrosis and contraction of the capsulorhexis after cataract extraction). There is also an increased risk of capsular and zonular tear.33 As with laser procedures, increased risk of IOP spike and increased inflammation during the postoperative period is possible. Topical steroids may need to be used for a longer period of time in these patients, as prolonged inflammation is common.34
Another consideration for patients with XFG is the role of minimally invasive glaucoma surgery (MIGS), although very little data is available. One study showed that Trabectome (MicroSurgical Technology) surgery is as effective in XFG patients as it is in POAG patients with low preoperative IOP, thin central corneal thickness and no history of SLT.35 Another study found that Trabectome surgery showed an overall greater IOP reduction to the mid-teens in XFG compared with POAG.36 As with all XFG treatments, the effects may diminish sooner than other types of glaucoma, resulting in a rapid IOP rise. Trabeculectomy and tubes have also been found to be as effective in XFG management as in POAG.37
| Arthritis Confounds Diagnosis A 44-year-old white male came to the office complaining of pain in his right eye. We last saw him years ago for bilateral uveitis that was controlled with topical steroids. At that time, we referred him to a rheumatologist who found no systemic etiology, but he did not return for subsequent eye care. He reported that his rheumatologist switched him from CellCept (mycophenolate mofetil, Genentech) to Humira (adalimumab, AbbVie). At his current visit, his best-corrected visual acuity was 20/30 OD and OS. Biomicroscopy revealed bilateral keratic precipitates with grade 2 cells OD and grade 1 cells OS. Intraocular pressures were 42mm Hg OD and 35mm Hg OS. OCT revealed inferotemporal thinning of the RNFL and the GCC OD while OS was normal. We diagnosed him with uveitic glaucoma OD and started him on 1% Pred Forte (prednisolone acetate, Allergan) 6x/day along with Cosopt (dorzolamide/timolol, Akorn) and brimonidine, both OU. We tapered his Pred Forte over several weeks. He is currently on brimonidine BID OU and his IOP is controlled in the low teens. |
Due to the genetic etiology contributing to the systemic disease of XFS, gene therapy is on the horizon for possible treatment modalities. Two single nucleotide polymorphisms (SNPs) on the LOXL1 gene account for nearly all XFS.30 Although not everyone with these SNPs will develop XFG, gene transfer by intraocular injection could be a viable option. This would represent the first treatment for glaucoma whose primary goal is to alter gene expression and not primarily decrease IOP. More study is needed on this exciting technology.
Pigmentary Glaucoma
Pigment dispersion syndrome (PDS) is a degeneration of the iris caused by the physical contact of lens zonules against the posterior iris surface, resulting in a mechanical liberation of pigment. This pigment in the anterior chamber of the eye can obstruct the trabecular meshwork drainage system, eventually leading to increased IOP and pigmentary glaucoma.
Potential ocular signs include mid-peripheral iris transillumination defects, corneal endothelial pigment and heavy pigmentation of the trabecular meshwork.
PDS has an estimated prevalence of up to 2.45% in the United States, with a higher incidence in men than in women.38 Men with PDS typically present in the third decade of life and women in the fourth decade. Myopia and white race are also risk factors for PDS. Genetic studies have determined a possible autosomal dominant inheritance pattern.39
A burn-out period can occur once the crystalline lens in the eye thickens with age, adjusting the zonular-iris contact area and preventing further pigment liberation.40 The probability of development of PG from PDS is approximately 15% at 15 years, with young myopic men most likely to develop PG.41
Once a diagnosis of PG has been made, treatment is similar to that of POAG, with therapy targeted at lowering IOP through topical treatment, laser or surgical procedures.
Other Secondary Glaucomas
In uveitic glaucoma, which affects up to 20% of patients with uveitis, the increase in IOP can be due to open-angle or closed-angle mechanisms.42
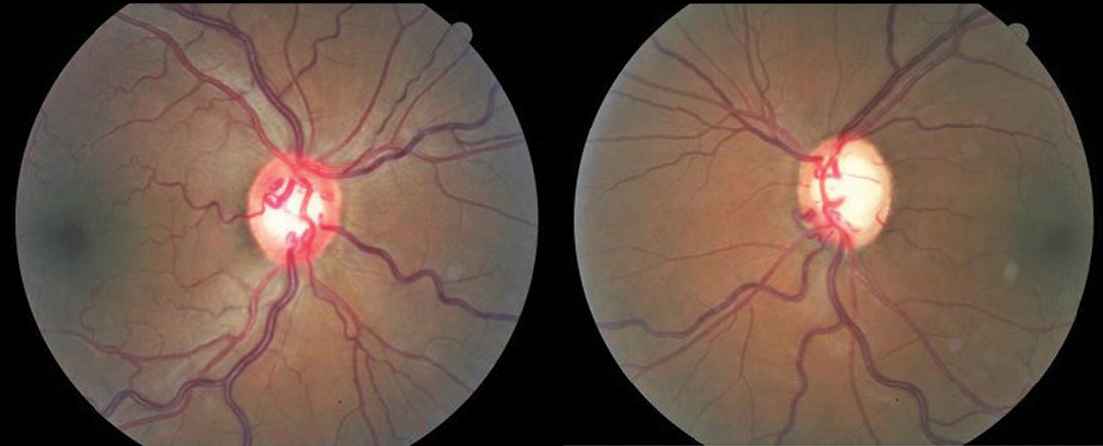 |
| Fig. 5. Asymmetric cupping in a PG patient with hypertensive retinopathy. Click image to enlarge. |
The onset with uveitis can be rapid via trabecular meshwork inflammation or chronic due to fibroblastic infiltration with subsequent scar tissue formation obstructing the anterior chamber angle. The patient can present with high IOP from these mechanisms or low IOP due to decreased aqueous production by the inflamed ciliary body. Keratic precipitates, band keratopathy, nodules, peripheral anterior synechiae (diagnosed by gonioscopy), posterior synechiae and eventual neovascularization of the angle can be noted, along with the typical cell and flare of uveitis.
| Optic Nerve Pathology in Chronic Angle-Closure Glaucoma A 61-year-old white female was referred with a diagnosis of chronic angle-closure glaucoma, OD>OS. She had bilateral peripheral iridotomies a year prior. She was being medicated with Lumigan ((bimatoprost ophthalmic solution, Allergan) QHS OU. Her IOPs were 25mm Hg OD and 21mm Hg OS. OCT revealed severe thinning of GCC and RNFL OD with minimal thinning OS. Visual fields were severely reduced OD and unremarkable OS. The right optic nerve exhibited significant cupping. Scheimpflug imaging revealed an anterior chamber depth of 2.35mm in the right eye with an angle opening distance at 700µm of 0.32 and 0.44 while the left eye was 2.39mm and 0.39 and 0.51, respectively (Figure 6). We added Combigan (brimonidine/timolol, Allergan) BID OU and scheduled her for cataract surgery plus Kahook Dual Blade goniotomy. |
Treatment is aimed at discovering the underlying cause of uveitis and lowering the acutely raised IOP (if present) to prevent further optic nerve head (ONH) damage. Topical IOP-lowering agents should be considered first. While prostaglandins can be used, proceed with caution due to their role in the inflammatory pathway.43
If IOP remains uncontrolled with topical treatment, surgical intervention (drainage implant or trabeculectomy) is needed.44 A recent study suggests Kahook Dual Blade (New World Medical) goniotomy might be helpful in managing these patients.45
Traumatic glaucoma often occurs due to recession of the anterior chamber angle after trauma. Angle recession is relatively common, with reports of it developing in up to 60% of eyes after non-penetrating or concussive trauma.46
Glaucoma can develop years or even decades after the initial injury. About 6.6% of eyes will develop glaucoma post trauma, which occurs more frequently in ocular contusions than in perforating injuries.47 Blunt force can cause an aqueous displacement, which eventually leads to tearing of the ciliary body muscles by traction on the iris root. Fibrotic tissue can then grow over the area of insult, leading to decreased outflow.48
While several factors can contribute to glaucoma developing in an angle recessed eye, increased degrees of recession (greater than 180 degrees) make glaucoma more likely. Other factors significantly associated with glaucoma following closed globe injury include increased pigmentation within the angle, elevated baseline IOP, hyphema and lens displacement.49
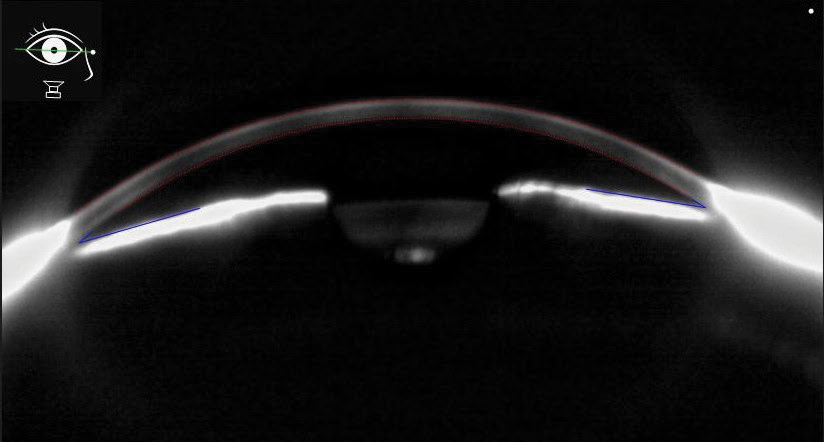 |
| Fig. 6. This Scheimpflug anterior segment image shows narrow angles in a chronic angle closure patient. Click image to enlarge. |
Gonioscopy is essential for examining the anterior chamber in search of areas of widening of the ciliary body band. Treatment is aimed at decreasing the elevated IOP to prevent ONH damage, and the clinical course can vary significantly depending on initial trauma. Topical IOP-lowering medications that suppress aqueous are preferred due to the likely compromise of the trabecular meshwork outflow pathway.50
Lens-induced glaucoma—both open-angle and closed-angle—can occur via several mechanisms. In phacolytic glaucoma, a rise in IOP with potential ONH damage can occur due to leakage of lens proteins of a mature cataract, causing an inflammatory reaction that leads to trabecular meshwork obstruction in an open angle.51 Closed-angle lens-induced glaucoma can occur when the swollen and hardened lens causes angle closure by proximity or lens dislocation (i.e., phacomorphic glaucoma).52
Removal of a hypermature cataract can pose several risks, including lens-particle glaucoma where capsular disruption leads to leakage of lens particles into the anterior chamber, altering normal aqueous outflow.53
| Optic Nerve Pathology in Chronic Angle-Closure Glaucoma A 61-year-old white female was referred with a diagnosis of chronic angle-closure glaucoma, OD>OS. She had bilateral peripheral iridotomies a year prior. She was being medicated with Lumigan ((bimatoprost ophthalmic solution, Allergan) QHS OU. Her IOPs were 25mm Hg OD and 21mm Hg OS. OCT revealed severe thinning of GCC and RNFL OD with minimal thinning OS. Visual fields were severely reduced OD and unremarkable OS. The right optic nerve exhibited significant cupping. Scheimpflug imaging revealed an anterior chamber depth of 2.35mm in the right eye with an angle opening distance at 700µm of 0.32 and 0.44 while the left eye was 2.39mm and 0.39 and 0.51, respectively (Figure 6). We added Combigan (brimonidine/timolol, Allergan) BID OU and scheduled her for cataract surgery plus Kahook Dual Blade goniotomy. |
While lens-induced glaucoma may not be common in industrialized nations, it poses a significant risk in developing countries. This secondary glaucoma affects up to an estimated 2.28 million in India, where the incidence of cataract cases far exceeds the total number of surgeries.54
Depending on the type of lens-induced glaucoma, several clinical findings may be observed. In phacolytic and lens-particle glaucoma, inflammation and white particles may be present in the anterior chamber along with corneal edema and synechiae. In phacomorphic glaucoma, a hypermature cataract is observed along with a closed angle and possible cell and flare.
Short-term treatment of this secondary glaucoma involves lowering the acutely raised IOP by topical medication (IOP-lowering medication and corticosteroids if inflammation is involved), with removal of the lens being the definitive treatment.
As optometrists continue to provide more glaucoma care, a proper understanding of the secondary glaucomas is paramount.
We have been trained to think that glaucoma is a long and slow process. But exfoliation glaucoma and pigmentary glaucoma can be aggressive. Aggressive glaucoma demands aggressive therapy.
Dr. Cymbor is a partner at Nittany Eye Associates in State College, PA. He is co-director of the Glaucoma Institute of State College and a member of the Optometric Glaucoma Society.
Dr. Stiles is a Captain in the US Army and currently practicing in the Midwest.
| 1. Wallace E, Lowry J, Smith SM, Fahey T. The epidemiology of malpractice claims in primary care: a systematic review. BMJ Open. 2013 July;3(7). 2. Tarkkanen A, Kivelä T. John G. Lindberg and the discovery of exfoliation syndrome. Acta Ophthalmol Scand. 2002;80(2):151-4. 3. Tarkkanen A. Exfoliation Syndrome: A Historical Perspective. J Glaucoma. 2018;27(Suppl 1):S1-S3. 4. Nazarali S, Damji F, Damji KF. What have we learned about exfoliation syndrome since its discovery by John Lindberg 100 years ago? Br J Ophthalmol. 2018;102(10):1342-50. 5. Arnarsson AM. Epidemiology of exfoliation syndrome in the Reykjavik Eye Study. Acta Ophthalmol. 2009;87(Thesis 3):1-17. 6. Katsi V, Pavlidis AN, Kallistratos MS, et al. Cardiovascular repercussions of the pseudoexfoliation syndrome. N Am J Med Sci. 2013;5(8):454-9. 7. Yildirim N, Yasar E, Gursoy H, Colak E. Prevalence of pseudoexfoliation syndrome and its association with ocular and systemic diseases in Eskisehir, Turkey. Int J Ophthalmol. 2017;10(1):128-34. 8. De Groef L, Van Hove I, Dekeyster E, et al. MMPs in the trabecular meshwork: promising targets for future glaucoma therapies? Invest Ophthalmol Vis Sci. 2013;54(12):7756-63. 9. Ritch R. Systemic Associations of Exfoliation Syndrome. Asia Pac J Ophthalmol (Phila). 2016 Jan-Feb;5(1):45-50. 10. Challa P. Genetics of pseudoexfoliation syndrome. Curr Opin Ophthalmol. 2009;20(2):88-91. 11. Genetics Home Reference. LOXL1 gene. NIH US National Library of Medicine. https://ghr.nlm.nih.gov/gene/LOXL1. Accessed October 25, 2019. 12. Wiggs JL, Pasquale LR. Expression and regulation of LOXL1 and elastin-related genes in eyes with exfoliation syndrome. J Glaucoma. 2014;23(8 Suppl 1):S62-3. 13. Dewundara S, Pasquale LR. Exfoliation syndrome: a disease with an environmental component. Curr Opin Ophthalmol. 2015;26(2):78-81. 14. Khawaja A. Pseudoexfoliative Glaucoma. EyeWiki. http://eyewiki.aao.org/Pseudoexfoliative_Glaucoma. Updated Septmeber 20, 2019. Accessed October 25, 2019. 15. Plateroti P, Plateroti AM, Abdolrahimzadeh S, Scuderi G. Pseudoexfoliation syndrome and pseudoexfoliation glaucoma: a review of the literature with updates on surgical management. J Ophthalmol. 2015;2015:370371. 16. Yaguchi S, Yaguchi S, Yagi-Yaguchi Y, et al. Objective classification of zonular weakness based on lens movement at the start of capsulorhexis. PLoS One. 2017;12(4):e0176169. 17. Fontana L, Coassin M, Iovieno A, et al. Cataract surgery in patients with pseudoex-foliation syndrome: current updates. Clin Ophthalmol. 2017;11:1377-83. 18. Calafati J, Tam DY, Ahmed IK. Pseudoexfoliation syndrome in cataract surgery. EyeNet. 2009;2009:37-9. 19. Fingert JH, Burden JH, Wang K, et al. Circumferential iris trans-illumination defects in exfoliation syndrome. J Glaucoma. 2013;22(7):555-8. 20. Rasmussen CA, Kaufman PL, Duehr PA, Bárány EH. The trabecular meshwork in normal eyes and in exfoliation glaucoma. J Glaucoma. 2014;23(8 Suppl 1):S15-9. 21. Desai MA, Lee RK. The medical and surgical management of pseudoexfoliation glaucoma. Int Ophthalmol Clin. 2008;48(4):95-113. 22. Kim S, Sung KR, Lee JR, Lee KS. Evaluation of lamina cribrosa in pseudoexfoliation syndrome using spectral-domain optical coherence tomography enhanced depth imaging. Ophthalmology. 2013;120(9):1798-803. 23. Tomaszewski BT, Zalewska R, Mariak Z. Evaluation of the endothelial cell density and the central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol. 2014;2014:123683. 24. Andrikopoulos GK, Alexopoulos DK, Gartaganis SP. Pseudoexfoliation syndrome and cardiovascular diseases. World J Cardiol. 2014;6(8):847-54. 25. Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124(5):685-7. 26. Gonen KA, Gonen T, Gumus B. Renal artery stenosis and abdominal aorta aneurysm in patients with pseudoexfoliation syndrome. Eye (Lond). 2013;27(6):735-41. 27. Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110(12):1752-6. 28. Cumurcu T, Dorak F, Cumurcu BE, et al. Is there any relation between pseudoexfoliation syndrome and Alzheimer’s type dementia? Semin Ophthalmol. 2013;28(4):224-9. 29. Elshafei AM, El-Badry MM. Association between pseudoexfoliation syndrome and sensorineural hearing loss. J Egypt Ophthalmol Soc. 2015;108(2):92. 30. Angelilli A, Ritch R. Directed therapy: An approach to the improved treatment of exfoliation syndrome. Middle East Afr J Ophthalmol. 2009;16(1):35-40. 31. Hodge WG, Damji KF, Rock W, et al. SLT vs ALT in patients with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2003;44(13):2183. 32. Holló G, Katsanos A, Konstas AG. Management of exfoliative glaucoma: challenges and solutions. Clin Ophthalmol. 2015 May;9:907-19. 33. Mohammadpour M, Erfanian R, Karimi N. Capsulorhexis: pearls and pitfalls. Saudi J Ophthalmol. 2012;26(1):33-40. 34. Sangal N, Chen TC. Cataract surgery in pseudoexfoliation syndrome. Semin Ophthalmol. 2014;29(5-6):403-8. 35. Tojo N, Abe S, Hayashi A. Factors that influence of trabectome surgery for glaucoma patients. J Glaucoma. 2017;26(9):835-44. 36. Ting JL, Damji KF, Stiles MC; Trabectome Study Group. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38(2):315-23. 37. Pelitli Gürlü V, Güçlü H, Özal A, et al. Comparison of long-term results of trabeculectomy to treat pseudoexfoliative glaucoma and primary open angle glaucoma. Int J Ophthalmol. 2018;11(1):66-70. 38. Lahola-Chomiak AA, Walter MA. Molecular Genetics of Pigment Dispersion Syndrome and Pigmentary Glaucoma: New Insights into Mechanisms. J Ophthalmol. 2018;2018:5926906. 39. Ramulu PM. Pigmentary glaucoma and Pigment Dispersion Syndrome. EyeWiki. http://eyewiki.aao.org/Pigmentary_glaucoma_and_Pigment_Dispersion_Syndrome. Updated June 11, 2019. Accessed October 25, 2019. 40. Hager J, Alward W. Pigmentary Glaucoma: 24-year-old male with episodic haloes around lights and blurry vision. EyeRounds.org. http://webeye.ophth.uiowa.edu/eyeforum/cases/184-pigmentary-glaucoma.htm. February 2, 2014. Accessed October 25, 2019. 41. Siddiqui Y, Ten Hulzen RD, Cameron JD, et al. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135(6):794-9. 42. Eliassi-Rad B, Francis A. Uveitic Glaucoma. EyeWiki. https://eyewiki.aao.org/Uveitic_Glaucoma. June 21, 2019. Accessed October 25, 2019. 43. Taylor SR, Gurbaxani A, Sallam A, Lightman S. Topical prostaglandin analogues and conjunctival inflammation in uveitic glaucoma. Open Ophthalmol J. 2012;6:75-8. 44. Kwon HJ, Kong YX, Tao LW, et al. Surgical outcomes of trabeculectomy and glaucoma drainage implant for uveitic glaucoma and relationship with uveitis activity. Clin Exp Ophthalmol. 2017;45(5):472-480. 45. Miller VJ, Young CE, SooHoo JR, et al. Efficacy of Goniotomy with Kahook Dual Blade in Patients with Uveitis-associated Ocular Hypertension. J Glaucoma. 2019;28(8):744-748. 46. Herschler J. Trabecular damage due to blunt anterior segment injury and its relationship to traumatic glaucoma. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(2):239-48. 47. Stanić R, Stanić R. Traumatic glaucoma. Coll Antropol. 2001;25(Suppl):101-4. 48. Brenner J. Angle Recession Glaucoma. EyeWiki. https://eyewiki.aao.org/Angle_Recession_Glaucoma. Updated August 30, 2019. Accessed October 25, 2019. 49. Sihota R, Sood NN, Agarwal HC. Traumatic glaucoma. Acta Ophthalmol Scand. 1995;73(3):252-4. 50. Clement CI, Goldberg I. The management of complicated glaucoma. Indian J Ophthalmol. 2011;59(Suppl):S141-7. 51. Dhingra D, Grover S, Kapatia G, et al. Phacolytic glaucoma: A nearly forgotten entity. European Eur J Ophthalmol. 2019:1120672119841972. 52. Mehta S, Hooda A, Mehta M. A study of clinical profile of phacomorphic glaucoma and its management outcome. Paripex-Indian J Res. 2019;8(3):13-14. 53. Luna G, Eliassi-Rad B. Lens Induced Glaucomas. EyeWiki. https://eyewiki.aao.org/Lens_Induced_Glaucomas. Updated June 21, 2019. Accessed October 25, 2019. 54. Peram V, Atti S, Paspula R, et al. Clinical types, IOP control and visual outcome in lens induced glaucoma. J Evol Med Dent Sci. 2015 Nov 16;4(92):15798-801. |
