 |
OCT Beyond the Basics: Unlock the Power of This Essential Tool
How to maximize use of this instrument based on the specific pathology at hand.
By Lee Vien, OD, and David Yang, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: March 15, 2024
Expiration Date: March 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists who want to learn about the impact this system has on practice.
Educational Objectives: After completing this activity, participants should be better able to:
Effectively use OCT scans and analyses in clinical practice.
Recognize the strengths and limitations of various OCT options.
Determine which type of OCT is the best option for various ocular pathologies.
Distinguish between newer technologies and understand the role of each.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Drs. Vien and Yang do not have nothing to disclose. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #127833 and course ID 89897-GO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
Optical coherence tomography (OCT) is an essential imaging test in the diagnosis and management of ocular pathology. It provides us clinicians with quantitative and qualitative information to help detect structural damage and is now used routinely to evaluate a variety of ocular conditions, including glaucoma and retinal disorders. Over the last 20 years, numerous multicenter, prospective clinical trials have employed OCT findings as their study endpoints. Their results have created new practice guidelines including the use of OCT to guide the management of retinal conditions such as diabetic macular edema, vitreomacular interface disorders and screening for hydroxychloroquine maculopathy.1,2
Although many optometric practices have an OCT, clinicians may not fully use their OCT instruments to obtain the best possible data when imaging a patient with ocular pathology. Providers often rely exclusively on preset scans provided by their instruments. These scans are adequate to screen for most suspected ocular pathologies. However, due to the fast acquisition and often low resolution of these scans, retinal pathology could be missed, particularly if the lesion is located between the displaced line scans or would be better visualized in a different orientation.
This article will walk through various ocular pathologies and discuss how to best use OCT scans and analyses to optimize clinical evaluations.
 |
|
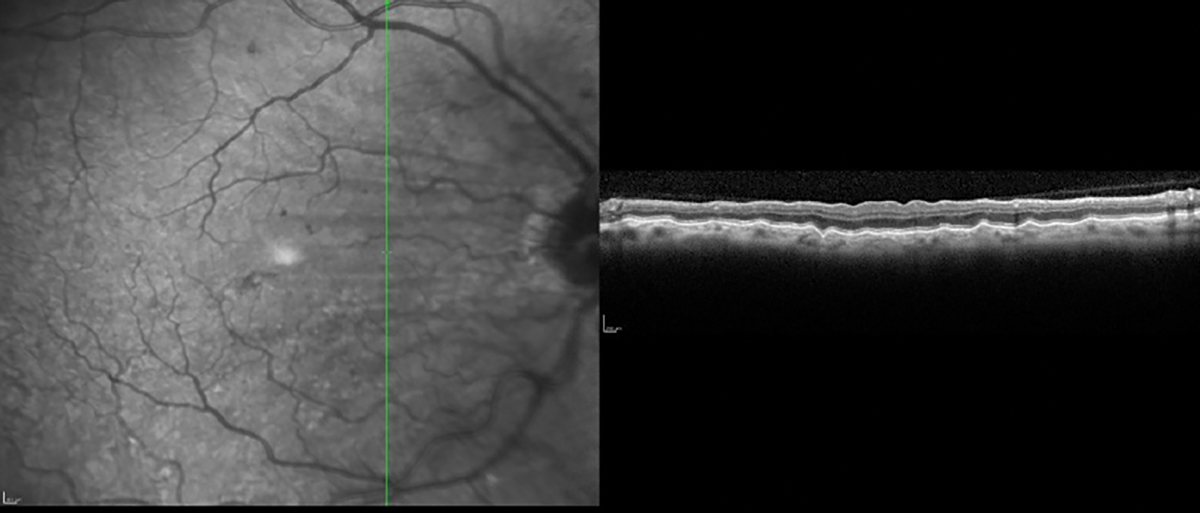
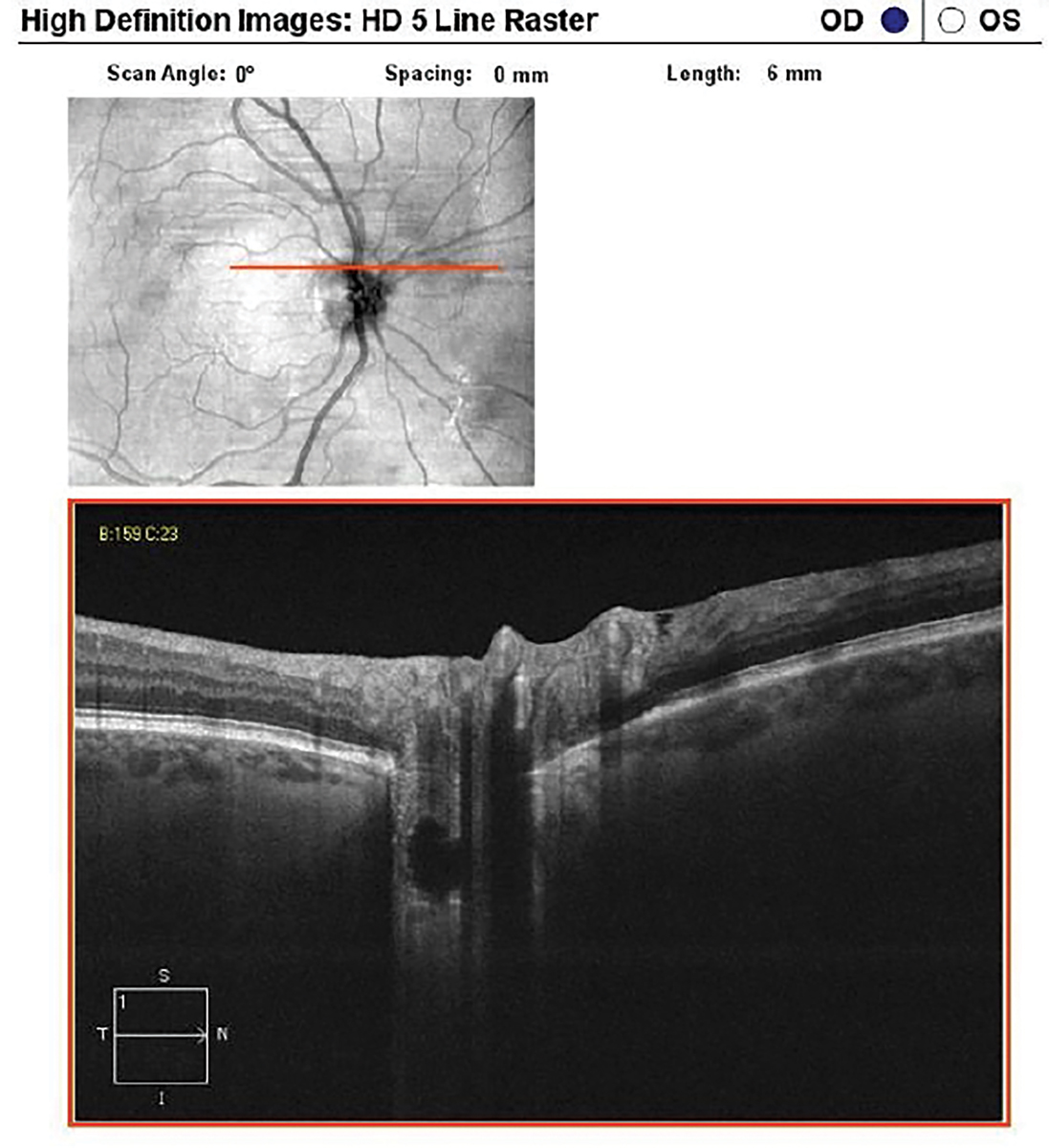
Choroidal folds imaged with high-resolution vertical line scan. Click image to enlarge. |
Choroidal Folds
Ocular pathologies present with a specific pattern of damage and using preset OCT scans may miss detection of that disease. An example is using preset scans with horizonal line cuts on a patient with choroidal folds. These phenomena are usually arranged parallel in a horizontal fashion, and therefore, using only OCT horizontal line scans may not detect the folds.3 To best detect horizontally oriented choroidal folds, multiple vertical high resolution line scans should be used. OCT instruments that generate data for both horizonal and vertical scans in a single acquisition can detect choroidal folds in different orientations. The folds can also be visualized on OCT instruments that provide a retinal pigment epithelium (RPE) layer map.
Hydroxychloroquine Maculopathy
High-definition scans are the top choice when detecting hydroxychloroquine toxicity to best visualize the interdigitation zone and ellipsoid zone. The initial paracentral area of involvement in hydroxychloroquine maculopathy may not be strictly nasal, temporal, superior or inferior. In a small study of hydroxychloroquine maculopathy eyes, it was found that the first area of parafoveal damage on SD-OCT was most often the inferotemporal quadrant.4 Therefore, horizontal or vertical line scans through the paracentral retina may miss early damage. Instead, a combination of horizontal, vertical and radial scans should be completed to screen for hydroxychloroquine maculopathy.
In a large multi-provider analysis on patients diagnosed with hydroxychloroquine maculopathy, more than 50% of Asian patients in the study had pericentral retinopathy without parafoveal involvement.5 To screen for pericentral retinopathy on OCT, wide-angle scans—such as 9mm or 12mm HD line scans in different orientations, especially out to the vascular arcades—should be used when accessible. If widefield imaging cannot be performed, scans can also be moved to accommodate pericentral lesions.
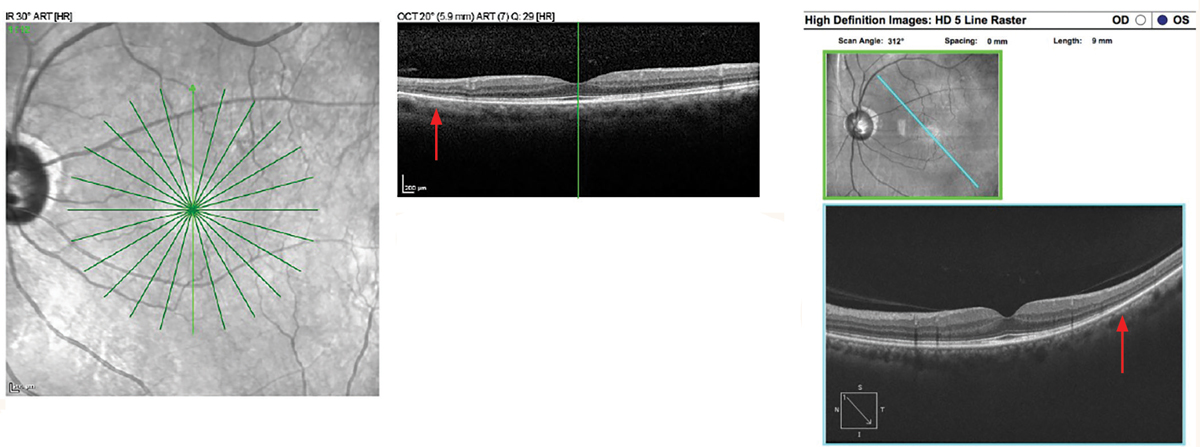 |
|
Hydroxychloroquine maculopathy with pericentral retinopathy. Spectralis SD-OCT Star scan (left image). Mild EZ disruption is present at the edge of the 6mm line scan inferiorly (top image). In the Cirrus HD 9mm line scan, note the loss of EZ inferotemporal in the pericentral retina (right images). Click image to enlarge. |
Pachychoroid Diseases
Enhanced-depth imaging (EDI) technology may not be a preset setting, but it is easily obtained using HD scans of the choroid. This type of imaging improves visualization of the choroidal structures and the sclerochoroidal junction. EDI is especially important in diagnosing pachychoroid diseases, such as central serous chorioretinopathy and polypoidal choroidal vasculopathy.6 Often, this feature is not turned on when running OCT scans and clinicians miss valuable choroidal details that can support the diagnosis.
The importance of differentiating pachychoroid diseases from age-related macular degeneration (AMD) is essential as the disease course and management differs between the two conditions. In pathologies such as adult-onset vitelliform macular dystrophy, a thicker choroid can help differentiate between this condition and AMD.7 In OCT devices without EDI, visualization of the choroid can be improved via a standard high-definition scan focused closer to the eye until the image is inverted or a scan done higher than the recommended reference lines.
Optic Nerve Imaging
When evaluating optic nerve disorders, clinicians often rely on the peripapillary retinal nerve fiber thickness (pRNFL), which is based on a set scan circle diameter that varies depending on the OCT instrument. The pRNFL provides valuable data. However, it can miss pathology that falls outside the scan circle. Some OCT instruments, provide not only pRNFL thickness but also a thickness map of the optic nerve and peripapillary area to aid in the detection of pathology outside the pRNFL scan circle. In addition, three-dimensional (3D) views of the optic nerve head (ONH) can be generated to image pathology that cause elevation or view the contour of the optic cup. OCT macula or volume scans can be placed over the optic nerve if specific optic nerve analysis software is not available. These 3D views and OCT B-scans over the optic nerve can highlight vitreopapillary adhesion when determining if a patient has a complete vitreous detachment.
 |
|
Chronic central serous chorioretinopathy with pachychoroid imaged with EDI OCT. There is a thin choriocapillaris and Sattler’s layer overlying the dilated Haller’s layer. Click image to enlarge. |
Optic Disc Pit and Lamina Cribrosa
The 3D views of the optic disc cube scan can also highlight the depth of the optic cup and reveal areas of suspected optic disc pits. Both congenital and acquired optic disc pits have a defect in the lamina cribrosa and the 3D scans will exhibit a deep optic disc cup over the area of the lamina cribrosa defect. High-definition line scans over the ONH with EDI or swept-source (SS)-OCT can improve visualization of the lamina cribrosa defect in optic disc pits. It is important to recognize optic disc pits because this condition can cause RNFL loss and visual field defects. It may lead to maculopathy from serous retinal detachment, retinoschisis and cystoid macular edema.8
Using EDI and SS-OCT, the lamina cribrosa has been studied extensively in glaucoma. Lamina cribrosa thickness has been found to have an association with glaucoma severity and is thinner in pseudoexfoliation glaucoma compared to primary open-angle glaucoma (POAG) with the same visual field mean deviation.9 Focal lamina cribrosa defects have also been found to be associated with glaucomatous damage and increase the rate of RNFL loss.10
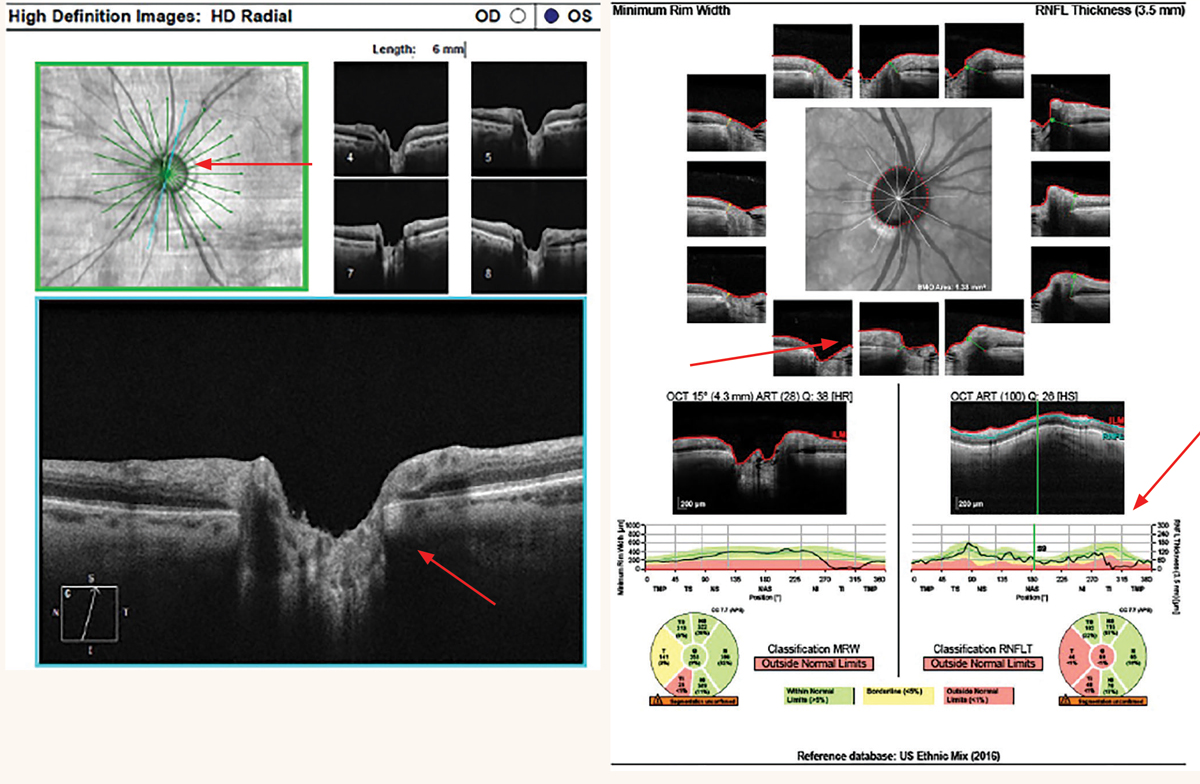
Neuroretinal Rim Thickness Assessment
In addition to pRNFL scans, the neuroretinal rim thickness can be evaluated with OCT scans over the ONH. OCT line scans in different orientations, especially aligned superotemporally and inferotemporally, can provide objective visualization of the neuroretinal rim thickness in glaucomatous eyes. A visibly thin neuroretinal rim thickness can be used to support an abnormal pRNFL thickness and clinical assessment of the ONH. This technique is helpful in eyes with obliquely inserted ONHs and tilted discs.
 |
|
Optic disc pit imaged with EDI OCT. There is a defect in the lamina cribrosa in the temporal optic disc with associated peripapillary retinoschisis. Click image to enlarge. |
The Bruch’s membrane opening (BMO) and minimum rim width (MRW) analysis provides quantitative and qualitative data of the neuroretinal rim tissue. The MRW is defined as the shortest distance between the BMO and the internal limiting membrane.11 Multiple radial line scans centered on the ONH are used to calculate the BMO-MRW, but note that each slice must be manually confirmed by the doctor to ensure the device accurately captured the BMO-MRW.
When comparing BMO-MRW to pRNFL thickness, some studies have found that BMO-MRW has better diagnostic accuracy than pRNFL, while others have reported no significant difference.12,13 Studies have also suggested that BMO-MRW is more sensitive for early detection of glaucomatous damage, but pRNFL may be more sensitive at monitoring glaucomatous progression.14 The best method to detect glaucoma and progression may possibly be the combination of BMO-MRW and pRNFL measures.15
Optic Disc Drusen (ODD)
It is important to differentiate between pseudo disc edema secondary to ODD vs. true disc edema, as the latter could be life-threatening. When imaging patients suspected of ODD, it is essential to acquire line scans with EDI SD-OCT or SS-OCT over the ONH. The latter uses a wavelength of 1300nm, longer than that used in posterior-segment SD-OCT and better for visualizing buried ODD.
On OCT, ODD has a hyperreflective margin with a hyporeflective core. Recent studies have also described peripapillary hyperreflective ovoid mass-like structures (PHOMS) that were previously considered to be a subtype of ODD. However, PHOMS is now believed to be axoplasmic stasis and nerve fiber herniation in the peripapillary region. These structures have been found to be associated with ODD, papilledema, anterior ischemic optic neuropathy, central retinal vein occlusion, optic neuritis and tilted disc syndrome.16
Peripheral Retina
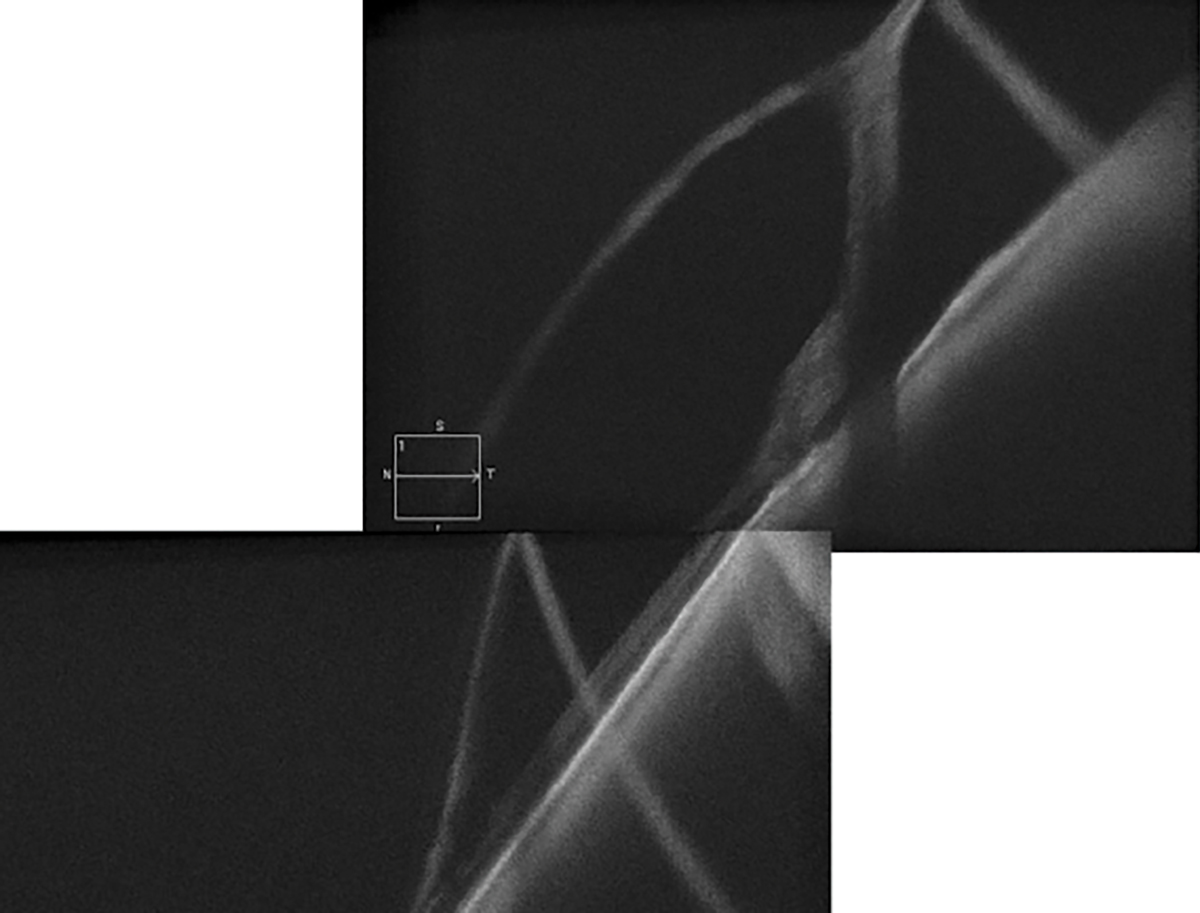
OCT imaging of the mid-peripheral and peripheral retina is now accessible with widefield imaging instruments and ultra-widefield (UWF) SS-OCT. Although these instruments provide high-resolution images of mid-peripheral and peripheral pathologies, they offer only line scans with no additional analyses, such as retinal thickness measurements.
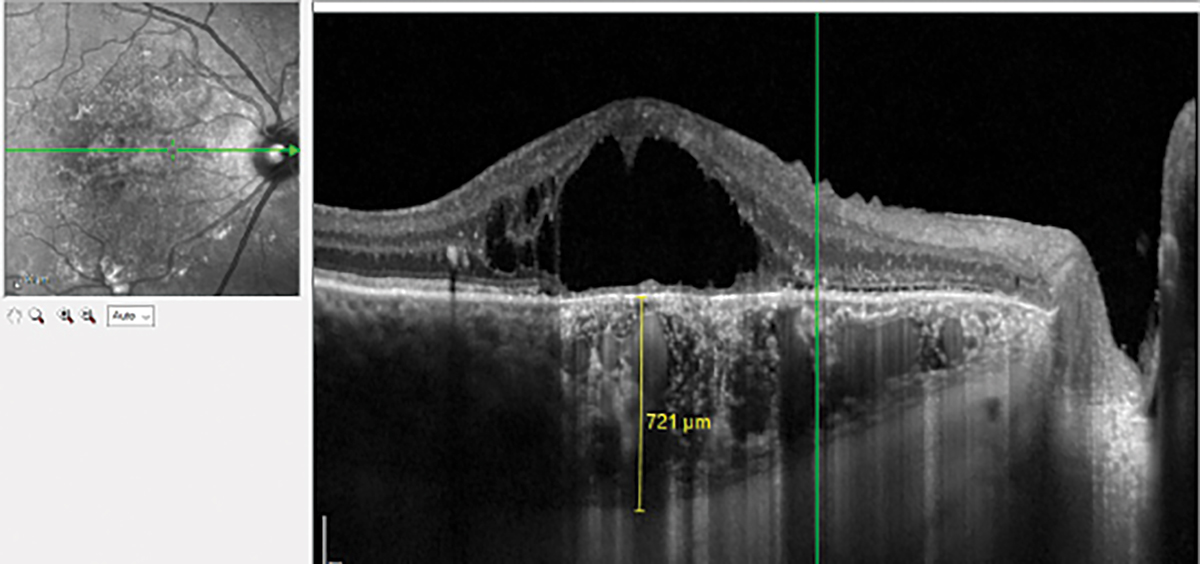
The good news is that even without widefield capabilities, standard SD-OCT 6mm line scans can assess mid-peripheral and peripheral lesions with the correct head positioning. In some OCT systems, these line scans can be extended to 9mm and 12mm. OCT imaging of the periphery has increased our understanding of peripheral retinal and choroidal lesions. It can help differentiate a retinoschisis from a retinal detachment and confirm shallow subretinal fluid in retinal tears and holes.
 |
|
At left: Cirrus radial 6mm line scans of a thin superotemporal neuroretinal rim from glaucoma. At right: Spectralis BMO-MRW of a thin inferotemporal neuroretinal rim from glaucoma. Click image to enlarge. |
Anterior Segment
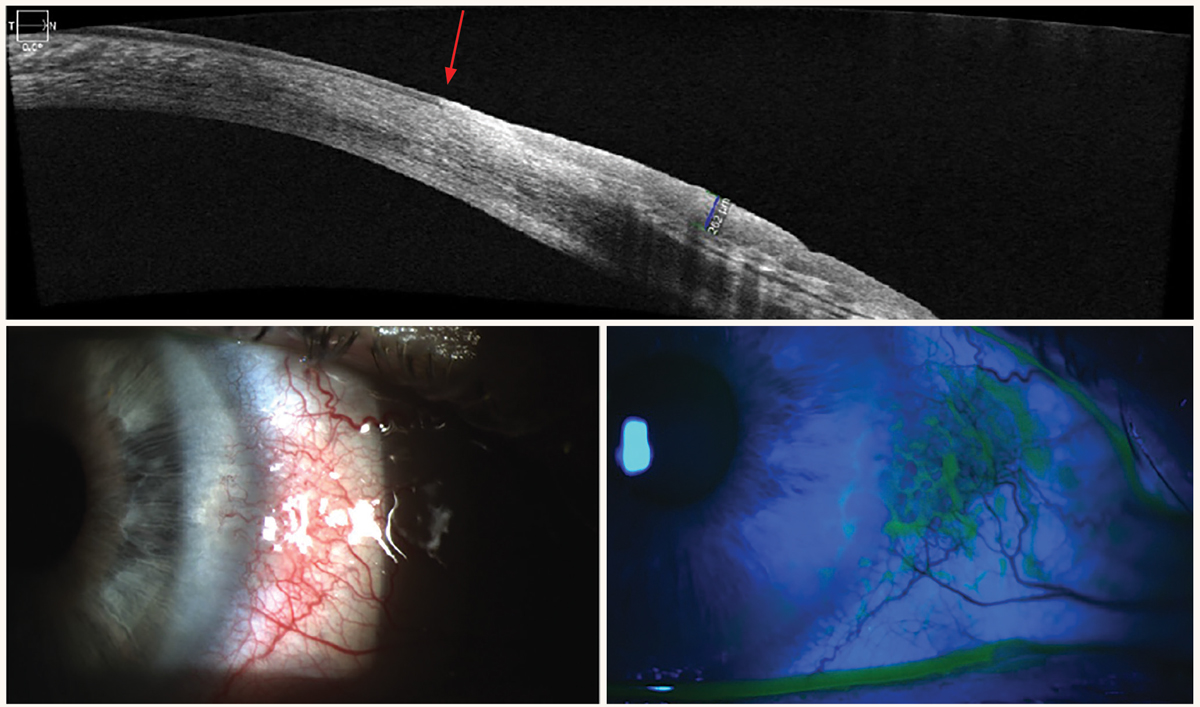
This imaging modality can provide identification and sequential evaluation of anterior-segment pathologies by capturing the anterior-chamber angle structures, cornea, conjunctiva, iris and lens. Anterior-segment OCT (AS-OCT) is now available on many SD-OCT instruments, so you likely won’t need to purchase a separate system. Many clinicians don’t take advantage of all their AS-OCT has to offer; for example, the technology can be used to image narrow angles, generate pachymetry maps, image ocular surface squamous cell neoplasia (OSSN) and most other anterior segment pathology, evaluate cells and flare in uveitis and assist in contact lens fits.
AS-OCT imaging of the iridocorneal angle requires no contact with the eye, can be performed by a technician and allows visualization of the angle under dark conditions. This is especially helpful in providing a quick evaluation of the iridocorneal angle in patients who cannot tolerate gonioscopy. It can also provide objective anterior-chamber angle parameter measurements using built-in tools. AS-OCT has been incorporated in large clinical studies, such as the Zhongshan Angle Closure Prevention (ZAP) trial. The ZAP trial found that AS-OCT biometric parameters of narrower horizontal angle opening distance from the scleral spur and flatter horizontal iris curvature were significantly associated with progression to primary angle closure (PAC) and acute angle closure.17
While AS-OCT can capture precise images of the iridocorneal angle, the technology does not replace gonioscopy. Limitations of AS-OCT compared to gonioscopy include poor visibility of the scleral spur in a number of eyes, static images of only certain quadrants in most AS-OCT instruments, inability to indent to assess for peripheral anterior synechiae or plateau iris and inability to assess the pigment in the angle. AS-OCT also has a high rate of false positives in the diagnosis of angle closure compared to gonioscopy.18
AS-OCT pachymetry maps provide non-contact central corneal thickness and topographical thickness maps to detect irregular corneal pathologies as well as aid in dry eye assessment. Pachymetry maps highlight areas of corneal thinning and can aid in the diagnosis of corneal pathology when a corneal topographer is not available. Epithelial thickness maps on AS-OCT have been shown to detect early keratoconus better than anterior corneal topography. This is due to the masking of the early ectasia from compensatory epithelial remodeling with thinning over the location of corneal steepening.19 On epithelial thickness maps, the epithelium will be thinnest over the area of the corneal steepening.20,21
 |
|
Buried ODD imaged with EDI OCT. Click image to enlarge. |
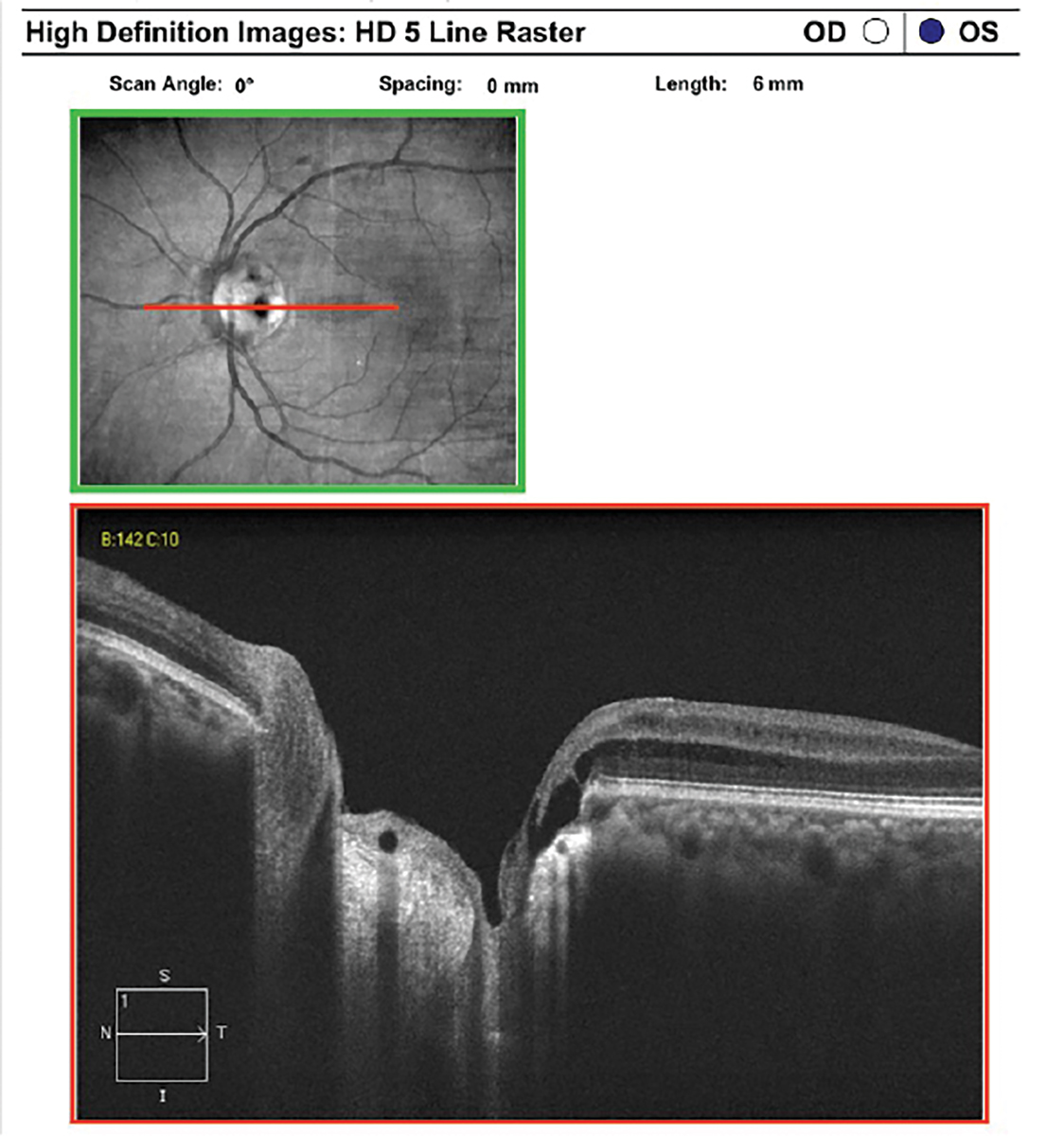
Another clinical advantage of AS-OCT is that it can provide an optical biopsy of abnormal conjunctival and corneal lesions. In OSSN, AS-OCT has distinctive features including a thickened and hyperreflective epithelial layer with an abrupt transition from normal to abnormal epithelium.22 AS-OCT attachments can also be used to discern between scleritis and episcleritis.
Newer Technology
Although most optometry practices have an SD-OCT, newer technology is continually being developed. It can be difficult to determine which upgrades are worth the cost. The upgrade could be software, but at other times may require a hardware upgrade or even a new OCT instrument if the technology is very different. Some of the newer technologies include AS-OCT, OCT-angiography (OCT-A) and SS-OCT.
AS-OCT has many applications in imaging anterior segment pathology. In SD-OCT instruments that have a built-in lens, a license upgrade is all that is needed to gain access to image the cornea and iridocorneal angle. Using a dedicated AS-OCT instrument may not be cost-effective in a primary care practice. Some disadvantages of AS-OCT are its limitation in penetrating the iris pigment epithelium and its lack of tracking and progression analysis. Furthermore, AS-OCT provides a static image, unlike gonioscopy that is dynamic and allows assessment of the pigmentation in the trabecular meshwork.
OCT-A provides noninvasive imaging of the retinal and choroidal vasculature. Since its introduction to clinical eyecare in 2014, there have been extensive studies evaluating its application in conditions such as diabetic retinopathy, AMD and glaucoma. In diabetic retinopathy, vascular changes in the foveal avascular zone can be detected on OCT-A before clinical findings of diabetic retinopathy are clinically detected.23 Details of the size and characteristics of macular neovascularization (MNV) from AMD have been studied on OCT-A, including nonexudative MNV.24 Peripapillary vessel density have been found to be lower in pseudoexfoliation glaucoma compared to POAG, suggesting a separate ischemic mechanism other than intraocular pressure.25
 |
|
Peripheral retinoschisis with outer retinal break and detachment imaged with HD line scan. Click image to enlarge. |
Although OCT-A provides valuable information, there are limitations. For example, it does not image leakage as seen on fluorescein angiography (FA) and is susceptible to artifacts such as motion. There is also a range of OCT-A instruments; some scan a wider area than others, some have faster scan acquisitions and only a few provide vascular density measurements.26 Without vascular density measurements, there is no quantitative data, making it difficult to track progression.
Even without OCT-A, structural OCT scans can map areas of poor perfusion due to retinal conditions. Areas of reduced capillary density on OCT-A in the superficial capillary plexus will correspond to areas of thin inner retinal layers on structural OCT scans. In exudative MNV seen on OCT-A, structural OCT will show subretinal or sub-RPE hyperreflectivity with subretinal and/or intraretinal fluid. In non-exudative MNV, structural OCT will exhibit a shallow irregular RPE elevation with a greatest transverse linear dimension of ≥1000µm, height of predominately less than 100µm and a nonhomogenous internal reflectivity.27
In clinical practice, the decision to treat using anti-VEGF in MNV from exudative AMD is often based on structural OCT due to its high sensitivity and specificity when compared to FA because not all practices have an OCT-A.28,29 Instruments with OCT-A also tend to be more expensive than those without it, and the purchase may be easier for optometry practices to justify if they see a large volume of posterior-segment diseases.
 |
|
OSSN on AS-OCT. There is a thickened and hyperreflective epithelial layer with an abrupt transition from normal to abnormal epithelium. Click image to enlarge. |
SS-OCT offers high-resolution imaging with fast image acquisition, allowing imaging of the choroid and vitreous simultaneously. The modality can penetrate lens opacities, choroid, pigment and blood better than SD-OCT due to its longer wavelength. Commercially approved SS-OCT instruments include the Optos Silverstone, Topcon DRI Triton, Zeiss Plex Elite 9000 and Heidelberg Anterion AS-OCT. Although the Topcon Triton has been FDA-approved since 2018, it has yet to be widely adopted in clinical practice compared to SD-OCT instruments. Currently, the Zeiss Plex Elite 9000 is mainly used in clinical research. The Optos Silverstone provides UWF OCT images but does not have capabilities for retinal thickness or pRNFL thickness analyses as do SD-OCT instruments. With newer advanced technologies (i.e., improved scanning speeds of 125 KHz on the Spectralis OCT-A module, analysis software and EDI), this modality still has a lot to offer, making an upgrade to SS-OCT less of a necessity.
Takeaways
OCT is a powerful tool that assists clinicians in diagnosing and managing ocular pathologies affecting the anterior and posterior segments of the eye. Using the appropriate testing strategies for the pathology of interest will allow for maximum utility of your instrument. Potential upgrades to OCT software and hardware that augment current features can also be considered to enhance patient care.
Dr. Vien is the director of optometry clinic operations and co-residency coordinator at the VA Palo Alto Healthcare System. He is an assistant clinical professor at Herbert Wertheim School of Optometry & Vision Science at UC Berkeley and Southern California College of Optometry at Marshall B. Ketchum University. Dr. Vien is also an affiliate clinical instructor at the Stanford School of Medicine.
Dr. Yang is the chief of the optometry section and co-residency coordinator at the VA Palo Alto Healthcare System. He is also a clinical professor at Herbert Wertheim School of Optometry & Vision Science and an affiliated clinical instructor at the Stanford School of Medicine. They have no financial disclosures.
1. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA Ophthalmol. 2019;321:1880-94. 2. Marmor MF, Kellner U, Lai TY, et al. American Academy of Ophthalmology recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-94. 3. Cangemi FE, Trempe CL, Walsh JB. Choroidal folds. Am J Ophthalmol. 1978;86(3):380-7 4. Marmor MA. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012;130:461-9. 5. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity.Ophthalmology.2015;122:110-6. 6. Castro-Navarro V, Behar-Cohen F, Chang Woohyok, et al. Pachychoroid: current concepts on clinical features and pathogenesis . Graefes Arch Clin Exp Ophthalmol. 2021;259:1385-1400. 7. Coscas F, Puche N, Coscas G, et al. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci.2014;55:64-69. 8. Georgalas I, Ladas I, Georgopoulos G, Petrou P. Optic disc pit: a review. Graefes Arch Clin Exp Ophthalmol 2011;249:1113 -22. 9. Moghimi S, Nekoozadeh S, Motamed-Gorji N, et al. Lamina cribrosa and choroid features and their relationship to stage of pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2018;59:5355-65. 10. Moghimi S, Zangwill LM, et al. Association between lamina cribrosa defects and progressive retinal nerve fiber layer loss in glaucoma. JAMA Ophthalmol. 2019;137(4):425-33. 11. Povazay B, Hofer B, Hermann B, et al. Minimum distance mapping using three-dimensional OCT for glaucoma diagnosis. J Biomed Opt. 2007;12:041204-8. 12. Torres LA, Sharpe GP, Hutchison DM, et al. Influence of Bruch’s Membrane opening area in diagnosing glaucoma with neuroretinal parameters from OCT. Am J Ophthalmol. 2019;208:94–102. 13. Wu Z, Vianna JR, Reis ASC, et al. Qualitative evaluation of neuroretinal rim and retinal nerve fibre layer on OCT to detect glaucomatous damage. Br J Ophthalmol. 2020;104:980-4. 14. Gardiner SK, Boey PY, Yang H, et al. Structural measurements for monitoring change in glaucoma: comparing retinal nerve fiber layer thickness with minimum rim width and area. Invest Ophthalmol Vis Sci. 2015;56: 6886-91. 15. Yang H, Luo H, Hardin C, et al. OCT structural abnormality detection in glaucoma using topographically correspondent rim and retinal nerve fiber layer criteria. Am J Ophthalmol. 2020;213:203-16. 16. Malmqvist L, Sibony PA, Fraser CL, et al. Optic Disc Drusen Studies Consortium. Peripapillary ovoid hyperreflectivity in optic disc edema and pseudopapilledema. Ophthalmology. 2018;125(10):1662-4. 17. Xu BY, Friedman DS, Foster PJ, et al. Ocular biometric risk factors for progression of primary angle closure disease. The Zhongshan angle closure prevention trial. Ophthalmology. 2021;129;267-75. 18. Desmond T, Tran V, Maharaj M, et al. Diagnostic accuracy of AS-OCT vs gonioscopy for detecting angle closure: a systemic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260:1-23. 19. Silverman RH, Urs R, Roychoudhury A, et al. Epithelial remodeling as basis for machine-based identification of keratoconus. Invest Ophthalmol Vis Sci. 2014;55: 1580-7. 20. Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604-10. 21. Kanellopoulos AJ, Asimellis G. Anterior segment OCT: assisted topographic corneal epithelial thickness distribution imaging of a keratoconus patient. Case Rep Ophthalmol.2013;4:74-8. 22. Thomas BJ, Galor A, Nanji AA, et al. Ultra high-resolution anterior segment OCT in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12:46-58. 23. Lu Y, Simonett JM, Wang J, et al. Evaluation of automatically quantified foveal avascular zone metrics for diagnosis of diabetic retinopathy using OCT angiography. Invest Ophthalmol Vis Sci. 2018;59:2212-21. 24. Laiginhas R, Yang J, Rosenfeld et al. Nonexudative macular neovascularization-A systemic review of prevalence, natural history, and recent insights from OCT angiography. Ophthalmol Retina. 2020;4:651-61. 25. Park JH, Yoo C, Girard MJ, et al. Peripapillary vessel density in glaucomatous eyes: comparision between pseudoexfoliation glaucoma and primary open-angle glaucoma. J Glaucoma. 2018;27:1009-16. 26. Spaide RF, Fujimoto JG, Waheed NK, et al. Optical coherence tomography angiography. Pro Retin Eye Res.2018;64:1-55. 27. Narita C, Wu Z, Rosenfeld P, et al. Structural OCT signs suggestive of subclinical nonexudative macular neovascularization in eyes with large drusen. Ophthalmology.2020;127:637-47. 28. Parekh PK, Folk JC, Gupta P, et al. Fluorescein angiography does not alter the initial clinical management of choroidal neovascularization in age-related macular degeneration. Ophthalmology Retina. 2018;2:659-66. 29. Gualino V, Tadayoni R, Cohen S, et al. Optical coherence tomography, fluorescein angiography, and diagnosis of choroidal neovascularization in age-related macular degeneration. Retina. 2019;39:1664-71. |
