Managing Uveitis With Steroids and Biologic Agents
The latest developments for this condition are based on underlying immunologic mechanisms. Here’s what that means for your practice.
By Jessica Steen, OD
Release Date: April 12, 2018
Expiration Date: April 12, 2021
Goal Statement: New research is lauding the potential of bioengineered complexes designed to cater to individual patients and alter their immune systems to respond to a variety of pathologies. This article explains how those techniques can target uveitis and how steriods can be used to help these patients; it also provides a detailed explanation of various types of uveitis and the mechanisms behind its development.
Faculty/Editorial Board: Jessica Steen, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 57601-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian and Michael Iannucci all have no relationships to disclose.
Uveitis is a manifestation of approximately 30 diseases, which are characterized by intraocular inflammation—rather than a single disease process.1-7 It can result from a wide range of underlying pathogeneses, such as infection, systemic disease or autoimmune dysfunction.1-9 While acute anterior uveitis is the most common presentation of intraocular inflammation we manage, chronic intermediate or posterior uveitis is less responsive to conventional therapy and has greater potential for long-term vision loss.1-8 The goal of treatment of all forms of uveitis is to achieve remission, with complete withdrawal of steroid and biologic agents, without relapse. This can be a challenge in chronic disease states.5,10 While each disease may have a specific therapeutic target, our classic treatment approach to all forms of uveitis—once infection is ruled out or controlled—is to broadly suppress inflammation with a single group of therapeutics.
 |
| This image show anterior vitreous cells in a patient with pars planitis. Click image to enlarge. |
The impressive therapeutic potential of biologic agents to treat a wide range of ophthalmic conditions is informing precision medicine in ophthalmic disease. Biologic agents are bioengineered complexes that alter expression of immunologic components of the immune system, including TNF-α inhibitors, anti-interleukins and interferon therapy.2,5,7 This article shows how an expanding appreciation of underlying pathogeneses of noninfectious uveitis—including improved understanding of genetic factors, cytokine expression and cellular characterization—is ushering in targeted biologic therapies for non-infectious uveitis.
Treatment and monitoring of patients receiving biologic therapy for uveitis requires a multispecialty approach.1,4,5 However, every optometrist should have a grasp of biologic therapy for the treatment of noninfectious uveitis to best prepare for comanagement.
Long-term Steroids
While topical steroids are typically sufficient to control and resolve acute noninfectious anterior uveitis, and may be beneficial in treating intermediate uveitis in pseudophakic, aphakic or vitrectomized patients, periocular and systemic steroids are often needed for treatment of intermediate and posterior uveitis.1-3,5,7
Periocular steroids, including intravitreal injections and sustained-delivery implants, are effective for suppression of intraocular inflammation due to non-infectious uveitis.10,11 Despite their efficacy, all steroids can cause local adverse effects that can include cataract formulation, elevated intraocular pressure (IOP), delayed healing and secondary infection development.1,5,10,11
Intravitreal steroid options include Kenalog (triamcinolone acetonide, Clint Pharmaceuticals) or Triesence (triamcinolone acetonide preservative-free, Alcon). While both are effective, only Triesence is approved by the FDA for intraocular use. Sustained-release intravitreal implants Ozurdex (dexamethasone 0.7mg, Allergan), Retisert (fluocinolone acetonide 0.59mg, Bausch + Lomb) and the insert Iluvien (fluocinolone acetonide 0.19mg, Alimera) may be effective for up to six months, 30 months and three years, respectively.10,11 While intravitreal steroids are effective in suppressing intraocular inflammation, the Multicenter Uveitis Steroid Treatment (MUST) trial determined that patients with chronic uveitis saw improved visual outcome with systemic immunosuppressive therapy compared with Retisert implant alone through seven years of follow up.12
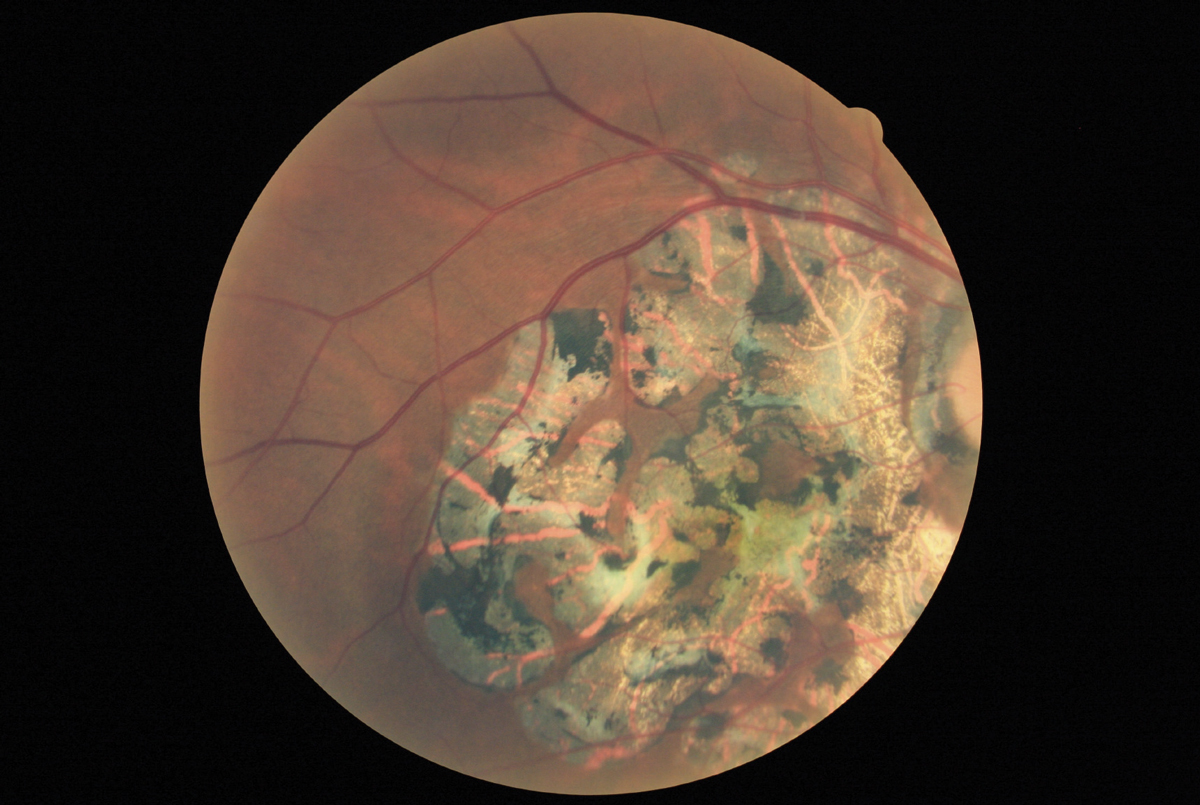 |
| This fundus photograph of inactive serpiginous choroiditis shows classic RPE and retinal atrophy. Click image to enlarge. |
In patients for whom inflammation cannot be controlled locally (a typical problem for those with posterior uveitis and panuveitis), systemic immunosuppression is necessary. Prednisone, given orally or intravenously, is effective for short-term control of inflammation.1,2,5,10 For patients with chronic uveitis that requires years of immunosuppression, significant systemic side effects, including elevated blood pressure, blood glucose dysfunction, gastrointestinal ulceration, fluid retention, osteoporosis and neuropsychiatric effects, make long-term treatment with steroids unpleasant—and typically unfeasible.10
Given the inability to treat chronic inflammation with systemic steroid agents, conventional non-corticosteroid immunosuppressive agents—also referred to as disease-modifying antirheumatic drugs (DMARDs)—or antimetabolites including methotrexate, azathioprine and mycophenolate mofetil have been used adjunctively to minimize systemic steroid treatment.5,7,8,10 In general, DMARDs are administered orally and act to suppress the entire immune system, as compared with biologic agents which are generally administered by subcutaneous injection or intravenous infusion and target specific locations within the inflammatory cascade.1,8,10 Effectiveness of conventional DMARDs may take months to determine.8,10,14 Each medication carries specific side effects, which include bone marrow suppression and liver toxicity.8,10 In rheumatologic disease, research shows, biologic agents such as TNF-α inhibitors and interleukin blockers are often more effective than traditional DMARDs alone.14
Contemporary management of noninfectious uveitis favors aggressive treatment early in the disease course, especially for chronic or relapsing uveitis cases.1
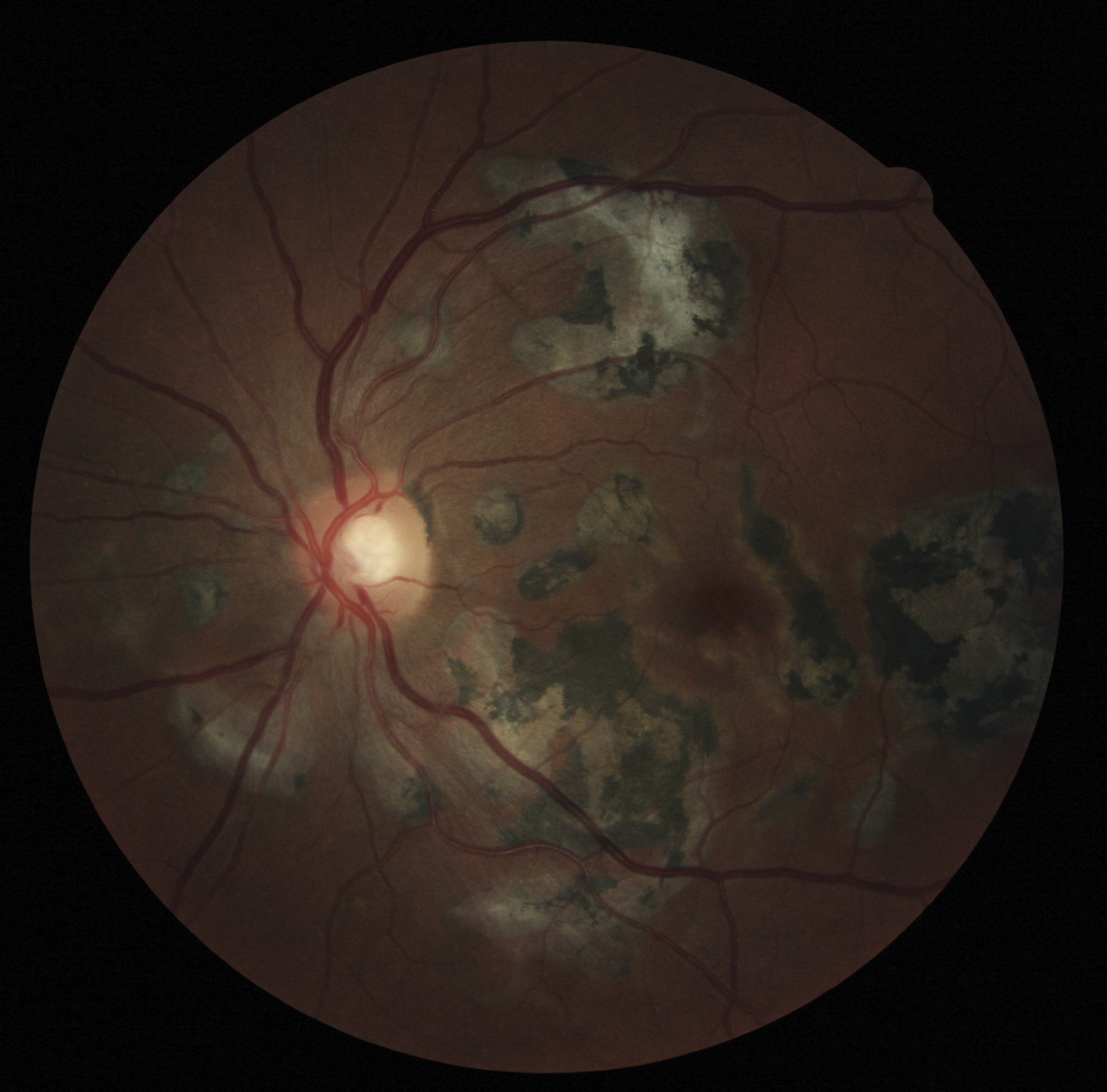 |
| Fundus photograph of inactive serpiginous choroiditis shows retinal atrophy and RPE hyperpigmentation. Click image to enlarge. |
Doctors frequently use biologic agents, either alone or in combination with steroids or traditional DMARDs, to control inflammation and to prevent associated vision-threatening consequences including macular edema, optic nerve disease or exudative retinal detachment.1,5,8,15
Biologic agents, while technically DMARDs, are bioengineered
complexes which target specific immunologic modulators, which are either up- or down-regulated in uveitis.2,5,7,8,10 Inflammatory components of the immune system—which include regulatory T-lymphocytes that produce cytokines such as TNF-α and interleukins—can be targeted at various levels to suppress downstream inflammation.2,5,6,10,16 Available treatments focus on inflammatory targets, which often overlap in uveitis cases.6,10,16
Biologic agents have been successfully used to treat rheumatologic conditions such as rheumatoid arthritis, plaque psoriasis, Crohn’s disease and ankylosing spondylitis; however, only a few specific groups of immune mediators are effective in uveitis treatment.8,10,14,17 All biologic agents currently available were developed for conditions other than uveitis, so the challenge to applying these therapies is determining the appropriate dosage for an adequate therapeutic response.2,8 A higher dosage at more frequent intervals is often required for control of uveitis.16
TNF-α Inhibitors
The most common biologic agents used in the treatment of uveitis are TNF-α inhibitors.1,2 TNF-α is a pro-inflammatory signaling protein, which is expressed in uveitis.8,10,16,18 TNF-α interacts with membrane receptors which, when activated, may signal apoptosis, activation or proliferation.16,18 In the normal state, typically no detectable level of TNF-α exists in the aqueous and vitreous humor.10,16 Elevated serum TNF-α is found in cases of inflammation—including inflammation due to trauma or immune-mediated processes.16 Currently available TNF-α inhibitors block various portions of the TNF-α pathway.10
Blocking a specific cytokine (e.g., signaling molecule), results in decreased immune response and often has secondary effects on other immunological pathways, which can cause significant adverse events.18 Adverse drug effects of TNF-α inhibitors include unmasking or inducing demyelinating disease, such as multiple sclerosis (MS), reactivation of latent tuberculosis, or viral hepatitis, auto-antibody formation and systemic lupus erythematosus.1,16,18 Additionally, the literature shows some controversy over a potential increased risk of lymphoma in patients with rheumatoid arthritis and pediatric Crohn’s disease.15
Prior to initiation of therapy, patients must undergo a complete physical evaluation to establish baseline organ function and rule out associated systemic inflammatory disease and infection.1,4 Risk of malignancy should also be assessed.1,15,29 Patients should have a baseline complete blood count with differentiation, purified protein derivative testing and chest radiograph to ensure no latent tuberculosis infection.4,15 MS can be exacerbated or induced by TNF-α inhibition.1 As pars planitis is associated with MS, pre-treatment magnetic resonance imaging (MRI) must be considered to rule out demyelinating disease, especially for patients with intermediate uveitis.1,6,15
While undergoing treatment with TNF-α inhibitors, serologic evaluation including a complete blood count with differential and metabolic panel, may be ordered monthly for the first three months of treatment and every two to four months afterwards.16
While many TNF-α inhibitors have been studied for the treatment of noninfectious uveitis, two common ones, Remicade (infliximab, Janssen Biotech) and Humira (adalimumab, Abbvie), have recently been recommended by an expert panel as first-line immunomodulatory
treatment in Behçet’s disease.18
Remicade is given as an intravenous infusion to suppress inflammation by binding to free and membrane-bound TNF-α to block activation, thereby reducing downstream inflammation.18,20 The majority of studies of infliximab in uveitis are in patients with JIA- and Behçet’s-associated uveitis due to the need for long-term treatment and the vision-threatening nature of the sequelae of chronic uveitis.8,18,21 In Behçet’s-associated uveitis, complete remission was achieved with infliximab alone in 86% of patients.1,5 Inflammation control was achieved in as little as two weeks, which was more rapid than treatment with intravenous corticosteroid or intravitreal triamcinolone alone.21
Humira is an FDA-approved biologic agent for the treatment of noninfectious uveitis and is indicated for intermediate, posterior and panuveitis in adults.18 Researchers believe adalimumab has less immunologic activity than infliximab due to its chemical structure. It blocks the inflammatory cascade by binding soluble and membrane-bound TNF-α.13,15,16,18 Off label, research shows an additional benefit in controlling anterior segment inflammation in patients with ankylosing spondylitis.17 Adalimumab is administered subcutaneously, although it is currently undergoing trials for intravitreal administration.23
Safety of treatment with TNF alpha inhibitors such as infliximab and adalimumab has not been evaluated in pregnant patients; however, they are considered to be pregnancy category B agents by the FDA.6,18
While TNF-α inhibitors share a similar mechanism of action, effectiveness in controlling intraocular inflammation of one agent does not necessarily mean that all agents will be equally effective.
Enbrel (etanercept, Amgen) is an isoform of the TNF receptor that inhibits inflammation through blockage of TNF-α and TNF-β from binding to the active TNF receptor.16,18 Although etanercept is effective in controlling inflammation in systemic conditions, it has shown no benefit in improving intraocular inflammation compared with placebo, and there is some, although limited, evidence that etanercept may induce uveitis and sarcoid-like disease.1,2,6,18
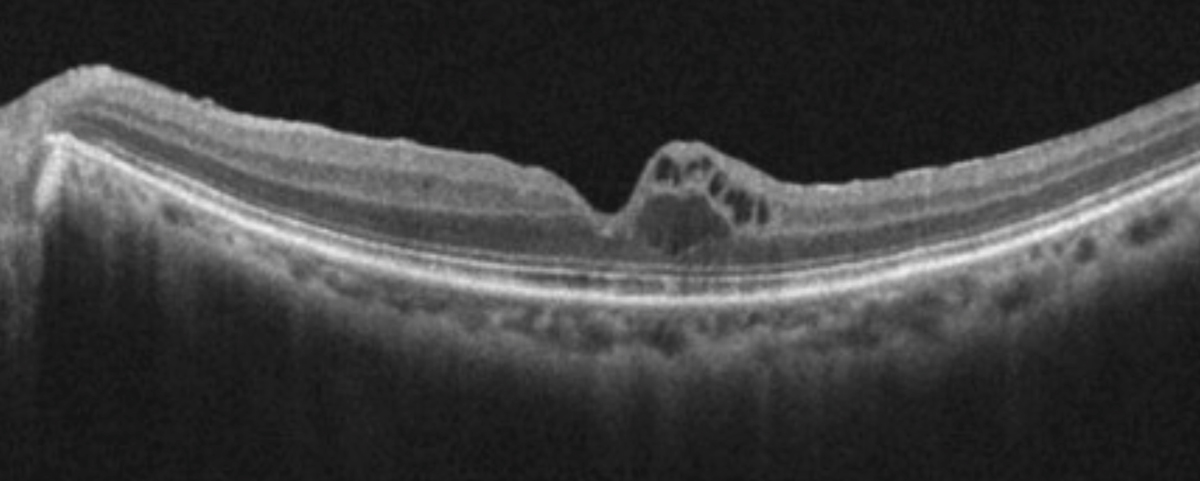 |
| Cystoid macular edema, seen here using OCT, is frequently observed in uveitis patients. Click image to enlarge. |
Non-TNF-α Inhibitors
Researchers are exploring non-TNF-α inhibitors such as Rituxan (rituxumab, Genentech), type I interferons and Orencia (abatacept, Bristol-Myers Squibb) for the treatment and management of noninfectious uveitis.
Rituxan is administered intravenously and binds to CD20 on B-cells, resulting in a combination of downstream effects including B-cell elimination, which permits their natural replacement.24 In addition to being approved for the treatment of certain forms of leukemia and lymphoma, it is often used as an off-label treatment for refractory cases of systemic lupus erythematosus and for uveitis due to juvenile idiopathic arthritis.6,24 Uncommon but potentially life-threatening side effects include progressive multifocal leukoencephalopathy, which results in progressive, irreversible white matter destruction.24
Type I interferons, including interferon alpha 2a, alpha 2b and interferon beta are effective in decreasing inflammation in Behçet’s- associated uveitis and intermediate uveitis associated with MS.5,10 Type I interferons increase the production of regulatory T-cells, which helps to keep the immune response in check.6 While interferon drugs may result in flu-like symptoms and depression, they are not associated with unmasking of demyelinating disease or reactivation of latent tuberculosis.5,10
Orencia is a biologic agent under investigation for the control of inflammation in uveitis. It suppresses inflammation by binding to CD80 and CD86 receptors on antigen presenting cells, which downregulates T-cell activation and reduces the immune response.6
Interleukin Blockers
Cosentyx (secukinumab, Novartis) is an interleukin blocker used for the treatment of psoriasis, ankylosing spondylitis and psoriatic arthritis.7 Secukinumab binds and blocks IL-17A, which is present in high levels in Behçet’s disease, but in low levels in healthy patients.1,25 Secukinumab is most effective intravenously, but it is not currently recommended for the treatment of non-infectious uveitis due to the limited data to support its use.1,25
Researchers are investigating other agents, including daclicizumab, gevokizumab and toclizumab, due to their theoretical potential to reduce inflammation in uveitis.2,26
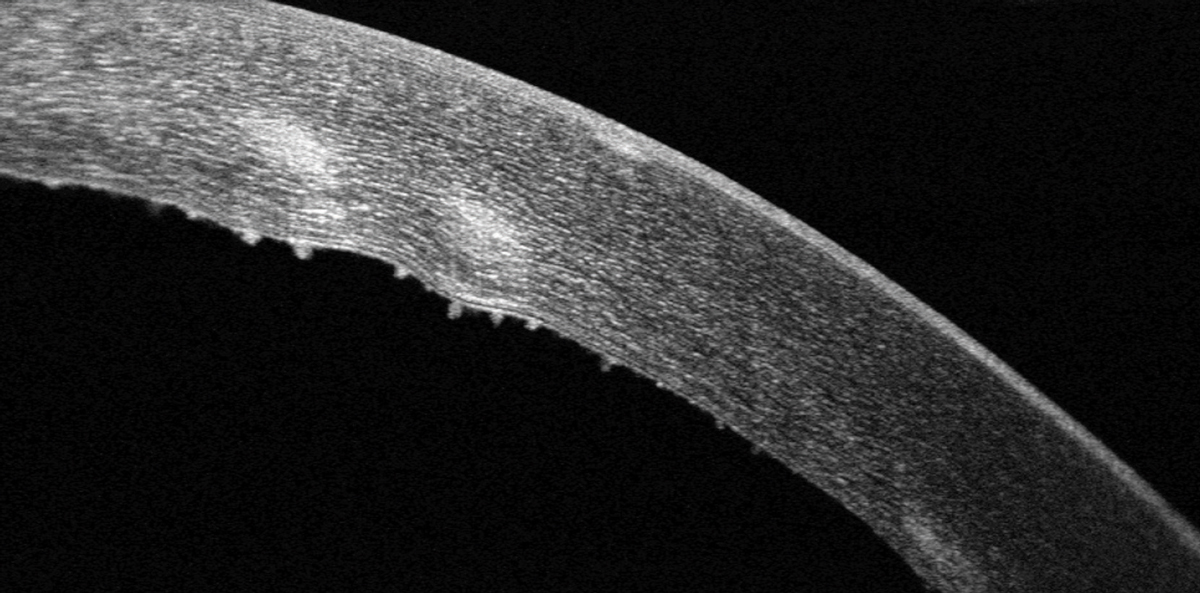  |
| Keratic precipitates are visible on the corneal endothelium with SD-OCT imaging. Click image to enlarge. |
Calling it Like it Is
A contemporary approach to uveitis diagnosis begins with a careful history and review of systems and a clinical examination that includes a dilated fundus examination and ancillary testing, when necessary.4
A proper dilated fundus examination can rule out masquerade syndromes and secondary causes of presumed inflammation.19 Many nonuveitic conditions can present clinically as a red eye with anterior chamber cell and flare, including leukemia and intraocular foreign body.4,19 Pigmented retinal epithelial cells in the anterior vitreous due to peripheral retinal break may appear as vitreous haze.19 Uveal melanoma can give rise to cells and flare due to breakdown of the blood-retinal barrier and leakage of proteins.4,19
The standardization of nomenclature in uveitis was developed to accurately and consistently describe features of uveitis amongst clinicians.27 It takes into account a description of the uveitis, including location of the primary site of inflammation, onset (sudden or insidious), laterality, duration (limited or persistent) and course (acute, chronic or recurrent) based on history of the condition and clinical characteristics.4,19,27
Sudden-onset uveitis is considered to be acute when a single episode is of limited duration, lasting less than three months.27 Chronic uveitis will relapse as soon as treatment is discontinued.4 In anterior uveitis, if topical steroid treatment is withdrawn, or tapered too quickly, a true acute form of uveitis may appear to be chronic.10
Intraocular inflammation is best treated aggressively, and topical ocular steroids should not be tapered until there is complete resolution of the inflammation—that is, no cells, flare or conjunctival hyperemia. Chronic disease is likely to have insidious onset and be persistent in duration.4,27 These particular cases typically require systemic immunosuppressive therapy due to the inability to control inflammation adequately with local treatment alone.4,10
Recurrent acute uveitis episodes are separated by a period of quiescence of at least three months before recurrence.4,10,27 Patients with recurrent, acute uveitis only require treatment during an active episode.4
Uveitis may be unilateral, alternating or bilateral.4,27 Identifying cases of bilateral asynchronous disease—where one eye is affected first, then the other becomes involved—is key as they are often associated with chronic ocular or systemic disease.4,27
Anatomic description of uveitis is based on the location of primary inflammation.4,27 In anterior uveitis, the primary site of iris and ciliary body inflammation is manifested in the anterior chamber, but there may be “spill-over” inflammation behind the lens into the anterior vitreous.27 Intermediate uveitis, which includes pars planitis, is characterized by signs of inflammation, primarily in the vitreous, clinically observed as vitreous haze.27 It is important to recall that inflammation can also “spill-over” from the vitreous into the anterior chamber. Findings common in anterior uveitis, such as posterior synechiae and peripheral anterior synechiae, are not likely to be found in intermediate uveitis.4,27 If synechaie are observed in what appears as an intermediate uveitis, there is likely to be a second, primary site of inflammation in the anterior segment. Posterior uveitis includes involvement of the retina and/or choroid and can be classified as retinitis, choroiditis, retinal vasculitis or neuroretinitis. Panuveitis includes inflammation throughout the eye.27
For anterior uveitis, clinical features revealed by a careful case history and close examination of the anterior segment will assist in making the correct diagnosis. For example, the presence of a hypopyon puts both Behçet’s disease and HLA-B27-associated uveitis high on the list of differential diagnoses.3,4,7 In patients with recurrent, acute onset unilateral or unilateral alternating uveitis, an underlying spondylarthropathy is likely.4,17 Heterochromia, iris atrophy and diffuse, stellate keratic precipitates will guide the diagnosis towards Fuch’s heterochromic uveitis.3,4
With a case description based on history, review of systems and clinical findings, we can guide our
laboratory evaluation, if necessary, from differential diagnosis.
Narrowing Down the Cause
Infectious causes of uveitis include tuberculosis, Lyme disease, cytomegalovirus, syphilis and herpes.3,4,6 Each has varied clinical features, relevant historical features and potential systemic findings that patients should be queried for through a careful history and targeted review of systems.4 Identification of an infectious cause will guide the course of treatment—which may include anti-inflammatory agents, including biologic agents, once infection is under control—to prevent inflammatory damage from the disease.1,8
Noninfectious causes of uveitis may be associated with systemic diseases including sarcoidosis, Behçet’s disease, Vogt-Koyanagi-Harada syndrome, ankylosing spondylitis and juvenile idiopathic arthritis (JIA).4,6,8,17,28,29 While angiotensin-converting enzyme (ACE) may be elevated in sarcoidosis, it has a low positive predictive value (22%), which limits its utility as a screening tool.4,29 A review of systems that focuses on specific organ systems including lungs, skin and reticuloendothelial system (liver, spleen, lymph nodes), will help to guide your diagnostic approach.4,29 In diseases such as Behçet’s disease and Vogt-Koyanagi-Harada syndrome, the diagnosis is made on clinical features, as no diagnostic laboratory studies exist. Risk factors for “masquerade syndromes” in uveitis should always be considered.2,4,19
In many cases of uveitis, an underlying cause will not be determined.1,4,6 After the completion of a careful clinical examination, ancillary testing and indicated laboratory work, in the absence of infection, neoplasm or systemic disease, uveitis may be determined undifferentiated.4 The traditional term, “idiopathic,” which we may be more familiar with, is a less precise descriptor. Undifferentiated noninfectious uveitis can be understood to be due to an inappropriate immune system response to an inflammatory trigger and, as such, is not truly idiopathic.4,6 Complete remission of undifferentiated forms of uveitis is possible through a presumed re-education process of the immune system.2,5,7
The diagnostic approach to uveitis should consider each form of the condition to be separate, but with overlapping features.4 Each case of uveitis should be approached carefully and systematically so focused laboratory or ancillary testing can be obtained when indicated.
Literature Prevalence
The literature evaluating the efficacy of specific biologic agents is dominated by two specific conditions: Behçet’s disease and JIA. These two conditions manifest quite differently, but share similar sequelae of uveitis including vision loss due to macular edema, choroidal neovascularization and hypotony.18,28,30,31 They both also require chronic immunosuppression due to the nature of disease. JIA-associated uveitis involves patients younger than 16, and Behçet’s disease uveitis typically affects working-age patients.28,30,31 As such, the need for effective, steroid-sparing therapies make biologic agents a promising group of treatments for the prevention of vision loss in the school and working population.
| Data Interpretation Challenges |
Behçet’s disease is presumed to be a result of a significant systemic inflammatory response.31 No specific laboratory test exists to confirm the diagnosis; rather, diagnosis is based on the presence of disease manifestations including oral lesions, urogenital ulcerations, skin nodules—most commonly on the lower extremities—and uveitis classically characterized with the presence of hypopyon.22,25,31 Uveitis associated with Behçet’s disease typically takes on a relapsing and remitting course that affects the anterior or posterior segment.18,22,25,31 A positive pathergy test, which indicates hypersensitivity to minor trauma, may aid in the diagnosis of Behçet’s disease; however, due to low sensitivity, especially in the North American population, a positive pathergy test is not required for diagnosis.25
JIA is a group of conditions that result in arthritis lasting more than six weeks in children younger than 16.18,28,30 The number of joints affected, presence of fever and skin rash and laboratory results help classify JIA.28,30 These conditions have varying serological findings
including potential rheumatoid factor positivity, HLA-B27 positivity and ANA positivity.28,30 Patients who have high ANA and who have an earlier age of onset of arthritis are more likely to develop uveitis.28,30
In JIA, the most common presentation of uveitis is bilateral insidious onset, chronic anterior uveitis.4,28,30 Treatment with biologic agents in uveitis patients has been frequently the target of study due to the condition’s profound potential for vision loss from secondary complications including macular edema, band keratopathy, uveitic glaucoma and hypotony, resulting in poor visual prognosis.18,28,30 As this condition manifests in childhood, vision loss has profound impact on the patient’s life.
Precision Medicine
Currently, systemic biologic agents are generally used off-label as second-line therapy for control of inflammation in patients with noninfectious uveitis who are not controlled with corticosteroids alone.1,7,10 Access to biologic agents can be challenging due to lack of FDA support for the majority of available treatments. General treatment trends in chronic uveitis are evolving from high-dose corticosteroids with delayed antimetabolites, to earlier adoption of biologic agents, with limited corticosteroid use.1,10
The best route of administration of biologic agents in chronic noninfectious uveitis, particularly intermediate, posterior and panuveitis, is still uncharted. While intravitreal or local therapy seems logical to ensure high-dose treatment to the specific structures affected and minimizing systemic effects, uveitis is considered secondary to an abnormal systemic immune reaction and, as such, systemic therapy may be necessary for effective treatment.6
Underlying pathogenesis of uveitis varies greatly. Specific immune targets may be central to the inflammatory cause in one disease state, but minimally important in another. The challenge is applying immunological mechanisms to complex, multifactorial diseases in an effort to achieve a predictable, repeatable response.16
While non-corticosteroid treatment options exist, many more are in the pipeline. For now, an informed individualized approach to the treatment of uveitis that best suits a particular patient’s disease course takes into account the cause of uveitis, systemic comorbidities and previous therapies. The not-so-distant future of uveitis management will involve a precision approach, which will focus on particular pathway targets in complex disease states in a specific genetic environment to determine optimal treatments.
Dr. Steen is an attending optometrist and assistant professor of ocular pharmacology at Nova Southeastern University’s College of Optometry.
1. Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis. Fundamentals of care for UveitiS (FOCUS) Initiative. Ophthalmology. 2018 Jan 6. [Epub ahead of print]. |
