Infiltrative Keratitis: Fight the Battle for Corneal Clarity
When infections invade the cornea and leukocytes gain ground, you need skillful strategy and carefully chosen weaponry.
By Jeffrey R. Urness, OD, and Chad E. Gosnell, OD
 |
Release Date: September 15, 2019
Expiration Date: September 15, 2022
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe the underlying difference in origin between sterile and infectious infiltrates (and ulcers).
- Explain the importance and process of history-taking, as well as the signs and symptoms, to help characterize the two different corneal presentations.
- Describe the “usual suspects” that may cause sterile and infectious infiltrates (e.g. CLPU, CLARE, EKC, microbial keratitis, etc.).
- Determine when to pursue further diagnostic procedures, such as culturing, and/or appropriate referral.
- Recognize which therapeutic strategy to employ, and when to employ it.
Target Audience: This activity is intended for optometrists engaged in the care of patients with corneal infiltrates.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Chad Gosnell, OD, Mann-Grandstaff VA Medical Center in Spokane, WA, and Jeffrey Urness, OD, Mann-Granstaff VA Medical Center.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 64411-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Drs. Gosnell and Urness have nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
The human cornea is unique among our body’s structures. It is designed for clarity, and its purpose is image formation, protection and beauty. Maintaining this corneal structure and function for a lifetime involves some impressive genetics, anatomy and physiology.
About 3,800,000 children are born in the United States each year, which means approximately 7,600,000 clear corneas begin their role to image a lifetime of vision.1 Though nearly all US infants begin life with a clear “window” to the world, some will, along life’s journey, be among the 30,000 to 70,000 US residents seen annually for microbial keratitis.2-4 Though a precise number is not known, more people seek care for non-infectious keratitis (sterile keratitis).3
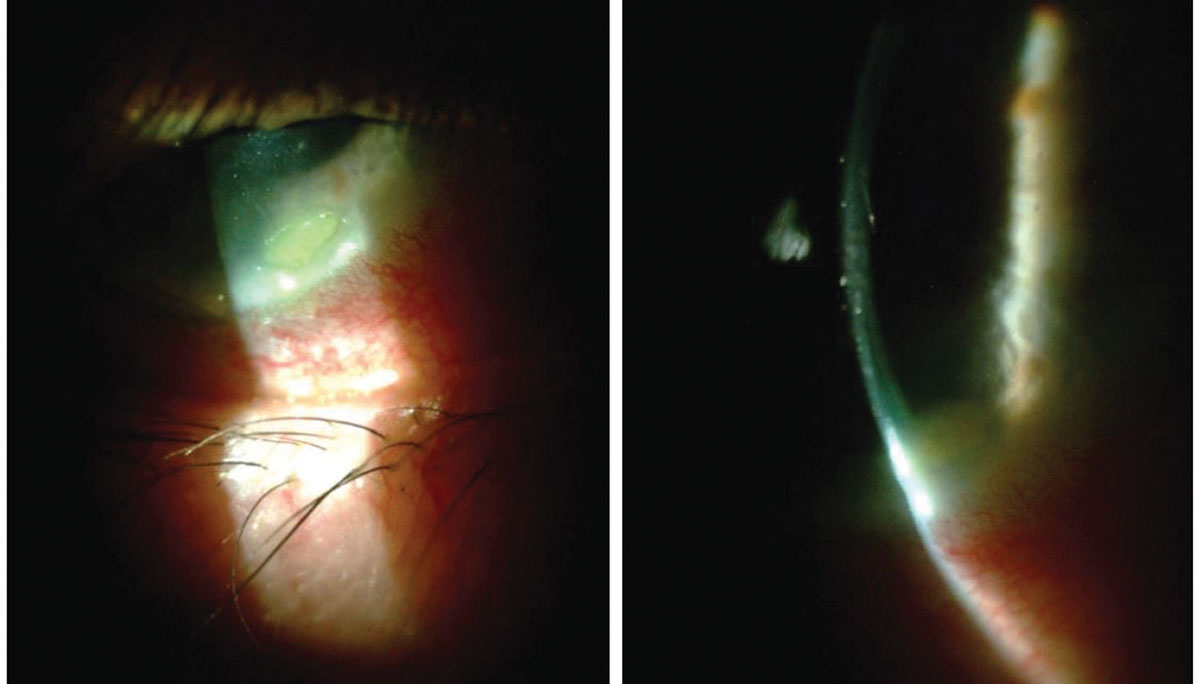 |
| A 70-year-old male with Staphylococcal hypersensitivity marginal keratitis presented with a peripheral infiltrate (with fluorescein staining) and adjacent conjunctival hyperemia. The optic section shows minimal overlying epitheliopathy. Click image to enlarge. |
The healthy cornea consists of a tear-coated, five- to six-layer epithelial covering, an approximately 500-layer “cross-woven” collagen core, and a single layer of endothelium. Apart from the sparse population of resident keratocytes, dendritic cells and leukocytes, the stroma is collagen and water.
The ocular surface has a multimodal defense design. First and most used is the innate biomechanical protection provided by the orbit, adnexal skin, eyelids, lashes, tear glands and tear film and conjunctiva and corneal epithelium. When healthy, these structures provide both static and dynamic biomechanical protection to the cornea and ocular surface.5 When this protection is insufficient or merely needs support, the innate immune system is mobilized to neutralize offending agents, remove injured tissue and restore any disrupted anatomy.6,7 Apoptosis and acute inflammation are two mechanisms of innate immunity serving to protect and restore ocular structure and function.7
The “first responders” of ocular innate immunity are local natural killer cells, macrophages and neutrophils, followed closely by dendritic cells.8 When corneal insult occurs, the complement system is activated and cellular cytokines and chemokines are released to recruit and guide immune cells in the immune-privileged cornea.9 The battle for corneal clarity ensues. This is the stage when leukocyte infiltration of the otherwise clear cornea begins.
When innate biomechanical and immune defenses need additional support, the third arm of protection is called to action: adaptive immunity. The macrophage and dendritic cells of the memory-less innate system serve to activate adaptive immunity. They bind foreign antigen, liberated by early invader destruction, and present them to local eye-related lymphoid tissue (lacrimal gland, conjunctiva and nasolacrimal mucosa), regional lymph nodes and, in some conditions, the spleen and thymus for generation of antibody-producing B-lymphocytes and priming of regulating T-lymphocytes, bringing both arms of adaptive immunity to the battle.7,10,11
Sterile vs. Infectious Infiltrates13 | |
| Sterile | Infectious |
| • Smaller lesion (<1mm) • <2 clock-hours extent • More peripheral • Minimal epithelial damage (defect size compared with underlying infiltrate) • No mucous discharge • Less pain and photophobia • Little or no anterior chamber reaction • No lid involvement | • Larger lesion (>2mm) • >2 clock-hours extent • More central • Significant epithelial defect (size of staining defect closely mirrors size of underlying stromal lesion) • Mucopurulent discharge • Pain and photophobia • Anterior chamber reaction • Lid edema, tear film debris, hypopyon |
Immune Privilege
Surrounding the visual pathway is anatomy and physiology that provide privileged, or “specialty,” immune regulation to protect the visual system from potentially self-destructive activity, while defending against “outsider” invasion. Cellular tight junctions, blood-brain barrier and immune downregulation are a few features providing this specialized protection to the eye and visual pathway.9 The design and organization of this protective system is greatly responsible for the varied clinical presentation seen in infiltrative keratitis.
Infectious infiltrative keratitis is defined as inflammation of the cornea due to attachment/invasion by viable microorganisms, while sterile infiltrative keratitis presents in corneal tissue devoid of live microorganisms. At times this distinction can be difficult to determine.10
Clinical appearance. The appearance of the cornea and surrounding structures yield valuable clues about the etiology and stage of disease. Larger, deeper, more central corneal infiltration with overlying epithelial destruction suggests microbial infection. The privilege of the central cornea and visual pathway provide greater opportunity for microbial attachment and invasion before the immune system “recognizes” the invasion.
In contrast, this paucity of central corneal immune cells supports tolerance to non-infectious forms of corneal insult. In the peripheral cornea and limbus reside numerous and diverse resident immune cells, which can respond quickly to local threats. In the case of microbes, innate immunity can quickly neutralize and eliminate invaders before they can become established. Non-infectious insult can trigger a rapid immune response, often generating characteristic peripheral corneal infiltration. For this reason, peripheral corneal infiltrates that are small, shallow, with minimal overlying epitheliopathy are more often sterile in etiology.
Empirical clinical exam guidelines, with slight variations, are well established to aid in determining whether infiltrative keratitis is sterile or infectious.12
Pertinent history. Sufficient history coupled with accurate observation is key to accurate assessment and effective treatment of infiltrative keratitis. The demographics and temporal descriptive history hold important clues to the absence or presence of infection.12 For example, a red, painful eye that develops shortly after a week’s vacation of hot tubbing supports suspicion for Acanthamoeba keratitis.
History of contact lens wear, pericorneal infections, recent eye trauma (including surgery), abnormal eyelid globe appositions, recent ocular exposure to contaminated water sources, concurrent head infection and relevant systemic conditions add to the risk and suspicion for infectious causes of infiltrative keratitis.
Detection of infection.Determining whether infection is likely is a high priority in the management of keratitis. The importance and nature of clinical workup and treatment differ based on any suspicion for infection, stage of disease and risk for visual compromise.
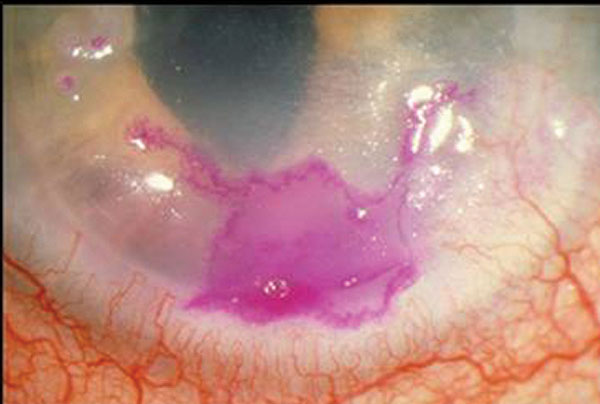 |
| Herpes simplex geographic keratitis, with the corneal lesion highlighted by rose bengal dye. Note that the geographic lesion exhibits border dendriform branches. Click image to enlarge. |
Historically and currently, culture and cytology are the most commonly employed ancillary tests used to improve detection of infectious etiologies; however, studies show that 38% of suspected corneal infections can be culture negative.4,12,14,15 Though positive culture, cytology or both support the identification of a causal microbe, correlation with history, clinical findings and clinical course are needed to support a diagnosis of an infectious etiology.12
Additional testing is sometimes employed to determine whether infection is present. This includes, but is not limited to, polymerase chain reaction (PCR), confocal microscopy and tissue biopsy.12,16-18 Familiarity with techniques (e.g., corneal sampling and biopsies) and co-participating professionals (microbiologists processing culture samples) improve the clinical benefit of results (fewer false outcomes).
Sterile infiltrative keratitis develops from a variety of corneal insults. Three conditions commonly seen in the optometric practice include hypersensitivity marginal keratitis, contact lens-related peripheral ulceration (CLPU) and contact lens-associated red eye (CLARE)
Bacterial Hypersensitivity Marginal Keratitis
Also known as sterile corneal infiltrates, this is a common non-infectious unilateral or bilateral immune response to uncontrolled bacterial blepharitis.19 Gram-positive Staphylococcus aureus is the most common offending species, although other bacteria have also been implicated.20
Signs and symptoms. Classically, this keratitis presents unilaterally or bilaterally with non-infectious infiltrates located at the 2, 4, 8 and 10 o’clock positions of the peripheral cornea.10These locations are fertile ground for this characteristic hypersensitivity immune response. First, the lid margins rest at these locations, promoting the highest concentration of bacterial exotoxins at these positions. Second, the close proximity of the limbal vasculature and eye-associated lymphoid tissue provides the resource for the immune response.
Eyelid-sourced bacterial exotoxins adhere to the corneal surface proteins, increasing corneal surface insult.21 Host antibodies develop and bind bacterial antigen, producing a type III hypersensitivity immune reaction.10 This signals the migration of polymorphonuclear leukocytes and fibroblasts to the site of the antigen-antibody complex. Once on site, these immune cells are positioned to protect the cornea from infection; however, their protective effort generates collagenase and proteoglycanase enzymes, producing collateral tissue damage and perpetuating corneal infiltration that can lead to ulceration.20
Typically, sterile marginal keratitis manifests with peripheral subepithelial infiltrates parallel the limbus and 1mm to 2mm from the corneoscleral junction. There is usually a corneal “clear zone” between the infiltration and the limbus. Infiltrates are characteristically circular and less than 2mm in diameter, but can coalesce into larger linear lesions.10,21 Less often, the infiltrate becomes circumferential in form.21,22
Frequently, corneal infiltrates express variable fluorescein staining, usually smaller than the underlying infiltrate. Mild, fine keratic precipitates and anterior chamber cells can accompany this keratitis. Another corneal finding seen with Staphylococcal lid disease is punctate epithelial keratopathy.23
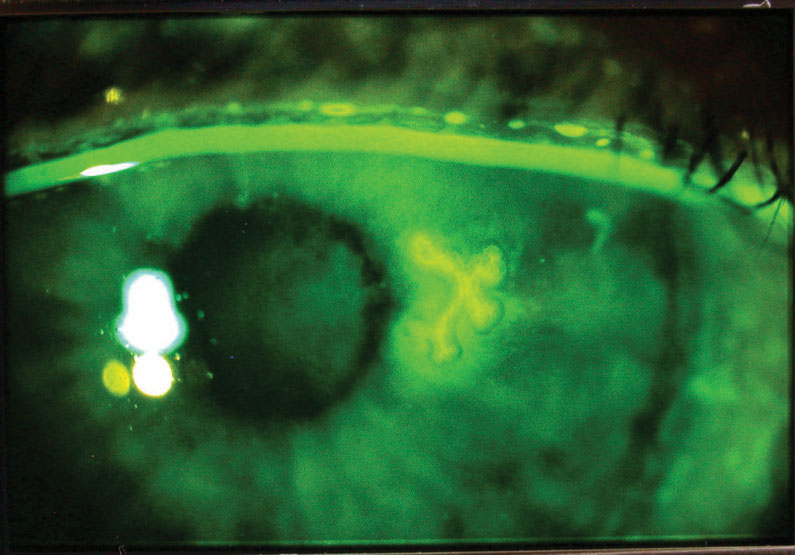 |
| Herpes simplex dendriform keratitis, with the corneal lesion highlighted with fluorescein dye. Click image to enlarge. |
Correlated signs of bacterial lid disease usually accompany hypersensitivity marginal keratitis.24 In some cases, facial signs of rosacea reveal the etiology, with blepharitis present in as many as 45% to 85% of rosacea patients.10
Symptoms from this sterile keratitis vary from mild foreign body sensation to mild pain. Patients often report light sensitivity, burning sensation, tearing, itch and excess mucin.19,24 Vision reduction is not a common feature of this disease.
Treatment. In general, management of infiltrative keratitis consists of two goals. First, the population of causal pathogens must be reduced, kept in check and hopefully eliminated. Second, the inflammatory tissue response should be modified and guided to resolution, minimizing permanent tissue damage. Depending on the severity of blepharitis and related keratitis, treatment usually involves eyelid hygiene as well as antibiotic and anti-inflammatory agents.
During the acute phase, eyelid hygiene should be performed morning and evening. The application of warm, moist compresses prior to lid cleaning, with a commercially available cleanser, will soften tissue production and facilitate biofilm reduction with subsequent cleaning.
Antibiotic application should follow eyelid hygiene and be directed at the lid margins. A broad-spectrum antibiotic agent is ideal when empirically treating blepharitis-related marginal keratitis. Clinicians should note that the antibiotic vehicle—drop vs. ointment—is important. Ointments are preferred due to ease of application and increased duration of effective contact with the eyelids and surrounding tissue.
Commonly used broad-spectrum antibiotic ointments are bacitracin or erythromycin. Because bacitracin is available only in topical form, less bacterial resistance has developed against it—an advantage over erythromycin. Bacitracin has also been shown to be more effective against methicillin-resistant Staphylococcus aureus, as well as outperforming the only fluoroquinolone ophthalmic ointment, ciprofloxacin.25-27 When you are confident the keratitis is sterile, the use of a topical corticosteroid will accelerate resolution of the keratitis and related symptoms.28
In severe and aggressive cases of marginal keratitis, clinicians should employ oral tetracyclines for their anti-inflammatory benefit, as well as their propensity to concentrate in the meibomian glands. Glandular concentration improves meibum quality while reducing pathogenic populations of offending bacteria.
Following antibiotic selection and dosing, anti-inflammatory treatment may be considered.20 When corticosteroid treatment is anticipated, infiltrates with overlying epithelial defects should prompt consideration for diagnostic cytology and cultures.10 If corticosteroids are instituted, their use should be preceded by effective broad-spectrum antibiotic therapy.10 This treatment approach requires prescribing independent antibiotic and corticosteroid agents. A mild steroid eyedrop such as fluorometholone or even a more effective steroid, such as loteprednol or prednisolone, may be selected depending on the severity of the inflammatory response.
Until the corneal epithelial defects are resolved and the keratitis is determined to be sterile, corticosteroid use should be “covered” by ongoing antibiotic treatment. If iritis is present, cycloplegia can add to symptomatic relief.
Another medication strategy widely used employs combination ointments (antibiotic + steroid). It is our position that this choice should be reserved for keratitis with intact epithelium and anticipated treatment course of seven to 10 days.
Some patients with staphylococcal hypersensitivity marginal keratitis should be followed every two to seven days until resolution is complete.19 With appropriate treatment and compliance, prognosis is good and improvement usually swift. Recurrences can be common and, as much as possible, patients and caregivers should understand the important role of eyelid hygiene in reducing future episodes.
Once the acute keratitis is resolved, expressive meibomian gland treatments may be beneficial for optimal control of causative eyelid disease. Low-dose maintenance therapy of oral doxycycline may be helpful, and can be used for months to years to reduce recurrences.10,19,20 If antibiotic ointments are chosen for long-term prophylaxis, consider monthly pulse treatments or cycling between different ointments to minimize resistance.
Contact Lens Peripheral Ulcer
CLPU is a common finding in hydrogel contact lens wearers. CLPU occurs at a rate of 7% per patient-year and is primarily associated with extended-wear contact lenses.29,30
CLPU is defined as an inflammatory reaction of the cornea to include corneal epithelial defect, infiltration, and necrosis of the anterior stroma.31 The pathogenesis is not well understood; however, it is thought the corneal infiltration is an adverse response to the Staphylococci species in the presence of contact lenses and epithelial defects. The corneal tissue is not infected with an organism, but rather is undergoing a sterile inflammatory response similar to that seen in marginal keratitis. A study in rabbit models showed that it is the corneal epithelial break that may allow the entry of bacterial exotoxins to the stroma and induce the infiltrative response.32
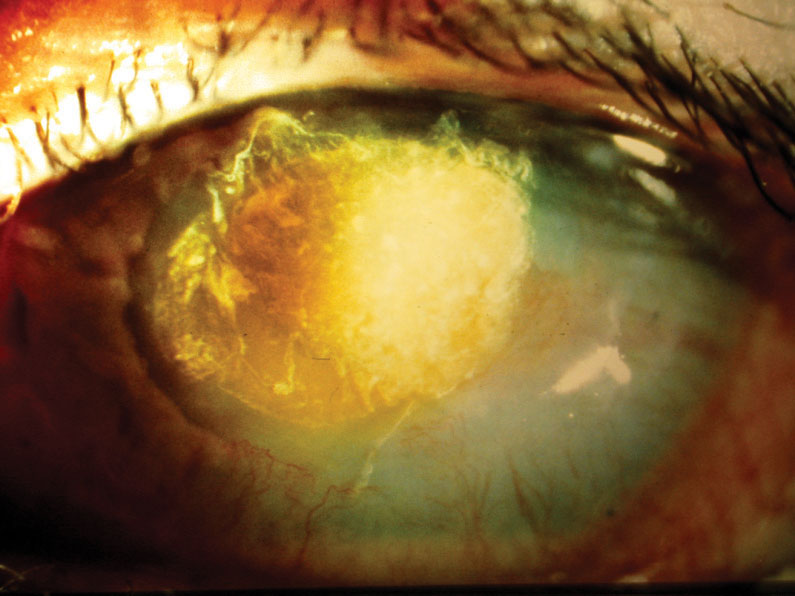 |
| A female patient in her late 20s developed Candida superinfection subsequent to culture-positive bacterial keratitis, which was initially responsive to a fortified topical antibiotic. Once the fungal infection was eliminated, the resulting scar required a corneal transplant, restoring acuity to 20/25. Click image to enlarge. |
Signs and symptoms. The conjunctiva is typically moderately hyperemic in the sector of the ulcer(s), though diffuse injection is common. Circular focal excavation with underlying infiltration and anterior stromal necrosis of the anterior stroma are located mid-peripheral to peripheral of cornea.31 Bowman’s layer is not breached, in contrast to microbial keratitis.29 CPLUs consistently stain with fluorescein. Patients experience moderate to severe pain or can be asymptomatic.
Treatment. This includes discontinuation of contact lens wear and the initiation of antimicrobial therapy until the corneal epithelium recovers from the ulcer. Typically, a fourth-generation fluroquinolone is used until re-epithelialization occurs.
As with marginal keratitis, once positive response to treatment is established, consider the addition of topical corticosteroids to reduce the inflammation and minimize scarring. Reduced epithelial defect size with smooth regular epithelial borders is an early finding with effective treatment.
If the infiltrate worsens or does not respond to treatment, further testing should include culturing, a second opinion or both.
Contact Lens-Induced Acute Red Eye
This is a common non-infectious infiltration of the cornea and inflammation of the conjunctiva, occurring at a rate of 11% per patient-year.29
CLARE is defined as an inflammatory reaction of the cornea and conjunctiva following eye closure.31 Typically, this occurs acutely following sleeping or napping in contact lenses. Risk factors for CLARE include extended wear contact lenses, high water contact lenses, tight fitting contact lenses, and recent upper respiratory infection.30
Signs and symptoms. Patients experience irritation to moderate pain, tearing and photophobia. Small, diffuse, mid-peripheral to peripheral corneal infiltrates will appear. No corneal fluorescein staining of the infiltrates is seen. There is moderate to severe circumferential redness.31
The pathogenesis of CLARE is similar to CLPU with no infected corneal tissue, but an inflammatory response to bacterial toxins. Additionally, there has been association between gram-negative bacteria and CLARE, including Haemophilus influenzae and Haemophilus parainfluenzae.30
Treatment. CLARE will self-resolve with discontinuation of contact lenses. In most cases, treatment includes not only discontinuation of contact lens wear but also copious lavage with artificial tears. In more severe cases, consider topical steroids four to five times per day with taper according to clinical course.31
The prognosis of CLARE is excellent. Scarring is rare. The infiltrates typically resolve in seven to 14 days without affecting visual acuity.31
When history and clinical features fail to convince the clinician that infiltrative keratitis is sterile, the search for an infectious etiology is necessary. There are four microbe classes that cause infectious keratitis: bacteria, virus, fungus and parasite.
Viral Keratitis
Viral infection is an intracellular invasion. The target tissue of attack and defense is cellular, producing infiltration of epithelium and/or endothelium along with adjacent stroma. The influence of immune privilege predisposes the central cornea to viral infectious infiltration (e.g., adenovirus and herpes virus).9 Though viral keratitis more often manifests in the central cornea, exception to such infection is seen in limbal keratoconjunctivitis.
Herpes simplex virus (HSV) is the leading cause of infectious monocular blindness.33 Currently, there is an upward trend of recurrent and new cases with approximately 60,000 active cases annually in the United States, resulting in nearly 1,000 corneal transplants each year.33
Dendriform herpetic keratitis is often readily diagnosed from appearance of epitheliopathy and infiltration; however, some hosts manifest non-dendriform disease. Geographic and limbal HSV keratitis are less common forms of disease that can be uncovered with corneal sensitivity and vital dye testing.
Correlating reports of pain with vital dye staining and corneal sensitivity testing can be helpful in suspected HSV keratitis. We have found off-label use of dental floss to be a good material for testing corneal and conjunctival sensation. If sterility is a concern, suture material is equally effective. Initial onset and early recurrence of HSV keratitis more often produces acute distressful pain.34 Damage to the sensory nerves with successive episodes tends to change and reduce the report of pain.
If HSV is suspected, but not evident based on history and appearance, ancillary studies (including culture, PCR and confocal imaging) can be effective.18
| What We’ve Learned From the SCUT Study Historically, the use of steroids in the treatment of infectious keratitis has been incorporated using “clinical wisdom.” More recently, research has been exploring the risks, benefits and optimal guidelines for treatment with ophthalmic corticosteroids. The recent Steroids for Corneal Ulcers Trial (SCUT) has often been cited in support of the safety of topical steroids in the treatment of confirmed drug-sensitive bacterial keratitis.12 For this study, empirical antibacterial treatment was initiated but a subsequent topical steroid was not instituted until culturing confirmed the presence of infectious bacteria and 48 hours of microbe-sensitive antibiosis had been delivered. The SCUT study supported the safety of topical steroid use when independent, non-combination antibacterial and corticosteroids were sequentially deployed in culture-positive drug-sensitive bacterial keratitis.12 If infection is believed to be present, yet the organism and its sensitivity have not been discovered, delay the use of steroids until empirical treatment appears effective and will eliminate the offending bacteria. Loading doses plus 24 hours of antibacterial medication will provide valuable information for the decision of corticosteroid use. One published study indicates up to 30% of infectious keratitis cases are polymicrobial.43 Polymicrobial infections are not exclusively bacterial. Clinical experience as well as research implicate greater challenge to treatment of polymicrobial infectious keratitis.43 Such cases often require poly-pharmaceutical intervention using fortified antibiotics. Because the SCUT study enrolled only cases of monomicrobial infection, it cannot be generalized to polymicrobial cases.12 |
Current treatment of active corneal herpes simplex infection involves antiviral medications either in topical or oral form and, in some cases, cycloplegia and topical anti-inflammatory medications. Though still used today, topical trifluridine is less often the drug of choice, giving way to newer and less toxic topical ganciclovir or oral antivirals (acyclovir, famciclovir or valacyclovir).23,34 When stromal disease presents, topical corticosteroids in addition to antiviral medication are indicated.33,36
In select cases of central corneal disease, epithelial debridement of viral-laden cells is indicated to accelerate removal of the immune stimulus. Following debridement, bandage contact lens and amniotic membranes in recalcitrant cases can be applied to facilitate re-epithelialization.35
The Herpetic Eye Disease Study (HEDS) found oral acyclovir treatment of epithelial keratitis to be equally effective as topical therapy, providing another option to minimize unintended effects and optimize compliance. (In patients with renal failure, topical therapy should be considered first.) The HEDS investigators found significant reduction in recurrence of both epithelial and stromal disease with oral antiviral prophylaxis; however, there was no reduction in the conversion from epithelial disease to the sight-threatening stromal form of the keratitis. The recurrence of stromal disease was reduced by about 50% over the year following prophylaxis with 400mg of oral acyclovir two times daily.33
Fungal Keratitis
In the western world, fungal keratitis is an infrequent intercellular opportunistic invasion of the cornea associated with contact lens wear, immune suppression and vegetative trauma.37 The incidence in the United States is uncertain, but is likely less than 2% of infectious keratitis cases, or about 30,000 cases/year.37,38
The most frequently detected fungi in keratitis are Fusarium, Aspergillus and Candida species.37 The prognosis for fungal keratitis is generally poorer than for other causative microbes, with perforation as high 50%.39
Biomechanical defense and innate immunity are the primary defenses against fungal keratitis.37 Macrophages and neutrophils are the early and predominant leukocyte responders to fungal invasion. Yield in fungal culture is low, so cytologic stains, confocal scanning and tissue biopsy can be valuable diagnostic adjuncts.37
In contrast to bacterial keratitis, fungal corneal infiltrates often have irregular feathery margins with satellite lesions. Early fungal infiltration often thickens the stroma and elevates compromised epithelium.37
Fungal keratitis frequently generates pain that is disproportionate to the acute inflammatory features, similar to many cases of Acanthamoeba infection.37 Contact lens wear, organic tissue injury and immune suppression/modulation are predisposing factors for fungal keratitis.37 Immune suppression not only adds risk for fungal infection, it facilitates bacterial and viral co-infection and complicates effective treatment.
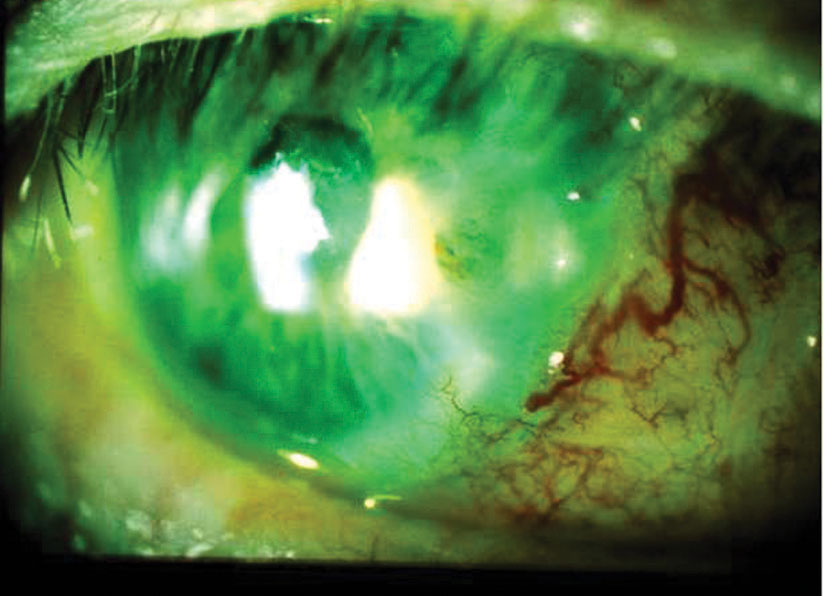 |
| After a pterygiectomy with graft, a male patient in his 70s failed to use his medications and ocular lubricants. By one week post-op, he had developed descemetocele with adjacent infiltrates and positive Seidel sign. Corneal culture was positive for Staphylococcus. Fortified antibiotics cleared the infection while a full-thickness button graft repaired the descemetocele. Click image to enlarge. |
From our experience, fungal keratitis frequently exhibits a gradual buildup of acute inflammatory signs, with corneal infiltration being a predominant finding. Fibrinous iritis and hypopyon are less common in early fungal keratitis.40
Infiltration from fungal infection requires access to the inter and sub-epithelial space, yet can progress beneath re-established epithelium.41 This re-epithelialization can impair drug penetration, thereby impeding sterilization of corneal tissues. Topical natamycin and amphotericin B are drugs often used in treatment of fungal keratitis. Natamycin remains the only commercially available ophthalmic drug FDA approved for treatment of fungal keratitis.42 Neither medication readily penetrates intact epithelium, requiring periodic epithelial debridement to maintain therapeutic drug levels at the site of infection. The dualistic fungal life forms (spore and hyphae) add to the difficulty of in vivo eradication, requiring longer treatment periods and often epithelial debridement to clear infection.
The toxicity of topical and systemic antifungal medications, along with extended course of drug delivery and corneal debridement, has historically prompted clinicians to have “definitive” cultures, cytology and/or biopsy before committing patients to the extended treatment needed to eradicate fungal keratitis. Recent studies looking at best practices for fungal keratitis management show that topical natamycin remains the most effective ophthalmic antifungal drug with the fewest adverse effects.37 Corneal crosslinking has yet to demonstrate added benefit to traditional pharmaceutical intervention.37,42
Bacterial Keratitis
Nearly all cases of infiltrative bacterial keratitis exhibit corneal epithelial compromise often exploited by contaminated contact lenses/solutions, eyelid disease, immunosuppression or prior ocular surgery, which can lead to infection.36 Staphylococcus, Pseudomonas and Streptococcus species remain the most frequently isolated bacteria.12
Once again, structure and function of ocular defenses increase the likelihood that larger and more central infiltrates have infectious etiology. Immune privilege, having its greatest impact farthest from the limbal cornea, gives greater opportunity for bacteria to exploit more central surface trauma, attaching, invading and populating central corneal tissue.
Subsequent immune signaling must call in surrounding resident leukocytes from lacrimal glands and eye-associated lymphoid tissue. The more intense and widespread the inflammatory symptoms and signs, the more likely bacterial infection is at play in infiltrative keratitis. Features of acute bacterial keratitis often include fast onset infiltrates 2mm in size with equal or larger overlying epithelial defects, greater than two clock-hours of extent involving mid-to-deep stroma with thinning and stromal necrosis.12 Hypopyon and fibrinous iritis should elevate suspicion for bacterial infection.
When faced with the possibility of bacterial keratitis, you must decide whether to pursue empirical treatment alone or ancillary test-guided intervention.
Empirical treatment. The advent of commercially available, broad-spectrum, highly effective topical fluroquinolones in the 1990s increased the practice of empirical treatment by non-corneal specialists.12 Patient history coupled with signs of sterile or mild infectious infiltrative keratitis often supports the decision for broad-spectrum topical fluoroquinolone treatment.
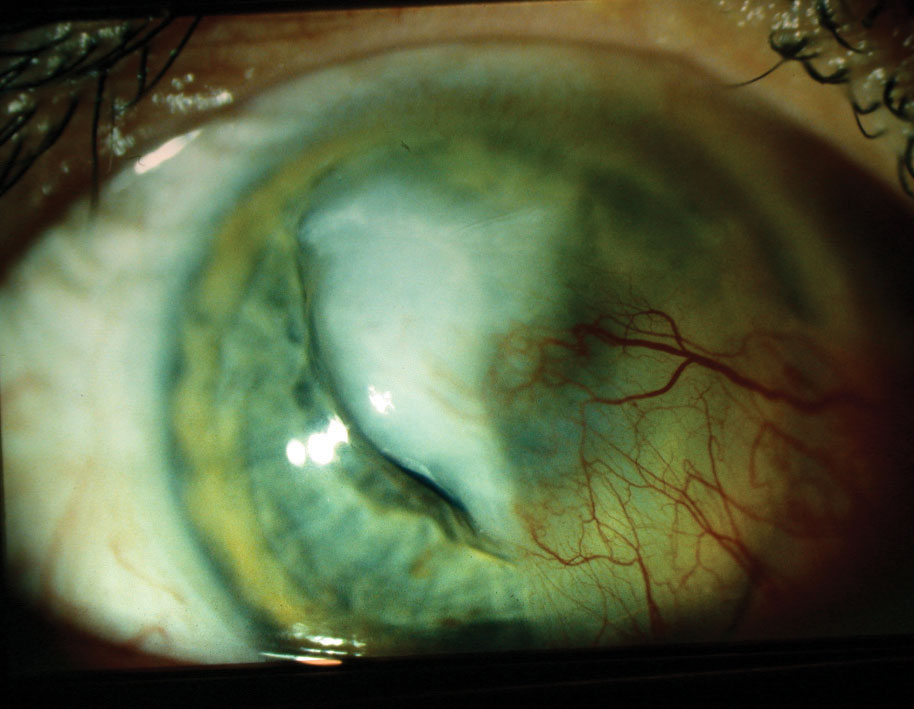 |
| A large central corneal infiltrate/ulcer with adjacent pre-existing pterygium, aggravated by culture-positive Streptococcus infection. Click image to enlarge. |
Test-guided treatment. When history and clinical presentation/course implicate moderate to high risk for sight loss, culture and cytology-guided management should be pursued.12 Culturing can be obtained using transport media or direct plating to culture media. The common transport media are Amies agar gel or modified Stuart’s medium.12 When “direct plating,” the most common media are blood, chocolate and Sabouraud agars.12 The clinician should sample eyelids along with the ulcerative infiltrative lesions. When there is history of contact lens wear, then lenses, cases and solutions should also be cultured.
If the initial treatment was empirical and clinical course appears unresponsive after 48 to 72 hours, discontinue empirical antibiotics for 24 hours and culture or refer to a corneal specialist before resuming anti-infective treatment.12
Depending on the location, extent and stage of corneal infiltration, debulking all necrotic epithelium, stroma and infiltration can improve efforts to sterilize the cornea and re-establish its structure. When debulking, be careful to avoid perforation. Prior to and following corneal sampling, the size of the corneal infiltrate(s) and any epithelial defect should be documented by diagram or imaging.
Managing keratitis can be straightforward, but should not be considered simple. The better the optometrist’s understanding of the various causations and impressively sophisticated immune responses, the greater the opportunity for rapid resolution of keratitis with minimal structural and functional damage.
Treatment of infiltrative keratitis often involves anti-infectives, anti-inflammatories and support therapies. The strength and dosing of needed medications should be employed with cautious confidence—confidence that comes from knowledge and experience. The use of anti-inflammatory and immune-suppressing corticosteroids is often beneficial, and frequently necessary, to achieve resolution of infiltrative keratitis. However, used indiscriminately or inappropriately, corticosteroid therapy can lead to poor outcomes with avoidable sight loss.
Knowing what medications to use at what strength for how long depends on the clinician’s understanding of the cause of the keratitis, its stage and the risk for permanent damage and sight loss. With this understanding, there is no need to surrender care when infiltrative keratitis comes to do battle.
Dr. Urness is a staff optometrist at Mann-Grandstaff VA Medical Center in Spokane, WA, and an adjunct clinical professor of optometry at Pacific University College of Optometry.
Dr. Gosnell is an optometrist at Mann-Grandstaff VA Medical Center and an adjunct clinical assistant professor at Pacific University College of Optometry.
|
1. Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final Data for 2017. National Vital Statistics Reports. 2018;67(8):3. 2. Hazlett L, Suvas S, McClellan S, Ekanayaka S. Challenges of corneal infections. Expert Rev Ophthalmol. 2016;11(4):285-97. 3. Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(45):1027-30. 4. Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022-8. 5. Akpek EK, Gottsch JD. Immune defense at the ocular surface, Eye (Lond). 2003;17(8):949-56. 6. Bolaños-Jiménez R, Navas A, López-Lizárraga EP, et al. Ocular surface as a barrier of innate immunity. Open Ophthalmol J. 2015 May;9:49-55. 7. Song J, Huang YF, Zhang WJ, et al. Ocular diseases: immunological and molecular mechanisms. Int J Ophthalmol. 2016;9(5):780-8. 8. Vemuganti GK, Reddy K, Iftekhar G, et al. Keratocyte loss in corneal infection through apoptosis: a histologic study of 59 cases. BMC Ophthalmol. 2004 Dec;4:16. 9. Hori J, Yamaguchi T, Keino H, et al. Immune privilege in corneal transplantation. Prog Retin Eye Res. 2019 Apr;S1350-9462(19)30001-1. 10. Krachmer J, Mannis M, Holland E (eds.). Cornea. 3rd ed. Philadelphia: Elsevier; 2011: 919-44, 1145-48. 11. Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45(3):722-7; 721. 12. Lin A, Rhee MK, Akpek EK, et al; American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Bacterial Keratitis Preferred Practice Pattern. Ophthalmology. 2019;126(1):P1-P55. 13. Stein RM, Clinch TE, Cohen EJ, et al. Infected vs sterile corneal infiltrates in contact lens wearers. Am J Ophthalmol. 1988;105(6):632-6. 14. McLeod SD, Kolahdouz-Isfahani A, Rostamian K, et al. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103(1):23-8. 15. Park J, Lee KM, Zhou H, et al. Community practice patterns for bacterial corneal ulcer evaluation and treatment. Eye Contact Lens. 2015;41(1):12-8. 16. Hau SC, Dart JK, Vesaluoma M, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94(8):982-87. 17. Alexandrakis G, Haimovici R, Miller D, Alfonso EC. Corneal biopsy in the management of progressive microbial keratitis. Am J Ophthalmol. 2000;129(5):571-76. 18. Kim E, Chidambaram JD, Srinivasan M, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol. 2008;146(5):714-23. 19. Gerstenblith AT, Rabinowitz MP, eds. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012:88-89, 125, 437. 20. Roy FH, Fraunfelder FW, Fraunfelder FT. Roy and Fraunfelder’s Current Ocular Therapy. 6th ed. Philadelphia: Saunders Elsevier; 2008:79-82. 21. Kanski JJ. Clinical Ophthalmology: A Systematic Approach. Edinburgh: Elsevier Butterworth-Heinemann; 2007:274-82. 22. Yanoff M, Sassani J. Ocular Pathology. 6th ed. Edinburgh: Mosby Elsevier; 2009:269-70. 23. Academy MOC Essentials: Practicing Ophthalmologists Curriculum 2017-2019 - Cornea/External Disease. American Academy of Ophthalmology; 2017;188-89. 24. Ganatra JB, Goldstein MH. Blepharitis. In: Yanoff M, Duker JS, eds. Ophthalmology. 3rd ed. St. Louis: Mosby Elsevier; 2008. 25. The Charles T. Campbell Eye Microbiology Lab. University of Pittsburgh School of the Health Sciences; 2019. http://eyemicrobiology.upmc.com/AntibioticSusceptibilities/Keratitis.htm. Accessed August 29, 2019. 26. Freidlin J, Acharya N, Lietman TM, et al. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthalmol. 2007;144(2):313-5. 27. Levinson BA, Rutzen AR. New antimicrobials in ophthalmology. Ophthalmol Clin North Am. 2005;18(4):493-509. 28. Abelson MB, Plumer A. Marginal infiltrates: A mysterious malady. Rev Ophthalmol. 2005;12(1):66-8. 29. Stapleton F, Ramachandran L, Sweeney DF, et al. Altered conjunctival response after contact lens-related corneal inflammation. Cornea. 2003;22(5):443-7. 30. Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84(4):257-72. 31. Sweeney DF, Jalbert I, Covey M, et al. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea. 2003;22(5):435-42. 32. Wu P, Stapleton F, Willcox MD. The causes of and cures for contact lens-induced peripheral ulcer. Eye Contact Lens. 2003;29(1 Suppl):S63-6. 33. Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000;118(8):1030-6. 34. White ML, Chodosh J. Herpes simplex virus keratitis: A treatment guideline – 2014. American Academy of Ophthalmology. June 2014. www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline. Accessed August 29, 2019. 35. Cheng AMS, Tseng SCG. Self-retained amniotic membrane combined with antiviral therapy for herpetic epithelial keratitis. Cornea. 2017;36(11):1383-86. 36. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678-89. 37. Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209-18. 38. Castano G, Mada PK. Fungal Keratitis. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; January 2019 www.ncbi.nlm.nih.gov/books/NBK493192. Accessed August 29, 2019. 39. Prajna NV, Krishnan T, Rajaraman R, et al. Predictors of corneal perforation or need for therapeutic keratoplasty in severe fungal keratitis: a secondary analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017;135(9):987-91. 40. Garg P, Rao GN. Corneal ulcer: diagnosis and management. Community Eye Health. 1999;12(30):21-3. 41. Tuli SS. Fungal keratitis. Clin Ophthalmol. 2011;5:275-9. 42. Prajna NV, Krishnan T, Rajaraman R, et al. Effect of oral voriconazole on fungal keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1365-72. 43. Lim NC, Lim DK, Ray M. Polymicrobial versus monomicrobial keratitis: a retrospective comparative study. Eye Contact Lens. 2013;39(5):348-54. |
