Dry Eye Disease: Know Your Comorbidities
Dry, irritated eyes can be one of the trickiest clinical findings. These systemic associations may be the key.
By Cecelia Koetting, OD
Release Date: November 15, 2018
Expiration Date: October 22, 2021
Goal Statement: Dry eye disease can manifest in just about any patient population with variable signs and symptoms, or none at all. Myriad systemic conditions can cause and contribute to a patient’s dry eye, and clinicians must have a basic understanding of many health arenas to properly diagnose dry eye and its etiology. This article discusses the current literature and mechanisms of action for many common systemic conditions that can contribute to dry eye to help clinicians better recognize patients presenting with multiple diagnoses.
Faculty/Editorial Board: Cecelia Koetting, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 59742-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The authors have no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Catherine Manthorp and Mark De Leon all have no relationships to disclose.
As a multifactorial disease affecting more than 30 million people within the United States, dry eye disease (DED) is an unavoidable clinical finding in optometric practice.1 Although race, sex and age are all factors that can affect a patient’s likelihood of DED, the condition can manifest in just about any patient population with variable signs and symptoms, or none at all. For example, studies have shown that women have a 50% to 70% increased risk of DED and worsening of signs—decreased tear osmolarity, Schirmer’s score and increased meibomian gland dysfunction (MGD)—particularly in postmenopausal women.1,2 Added to this complex clinical picture is the association between DED and many systemic conditions such as diabetes, inflammatory diseases, thyroid dysfunction and dermatological, psychological and neurological diagnoses. With myriad comorbidities causing and contributing to a patient’s dry eye, clinicians must have a basic understanding of many health arenas to properly diagnose and manage the disease.
This article highlights many common systemic conditions that may be contributing to your patient’s dry eye. A brief discussion of the current literature and mechanisms of action will help clinicians better recognize patients presenting with multiple diagnoses contributing to one another.
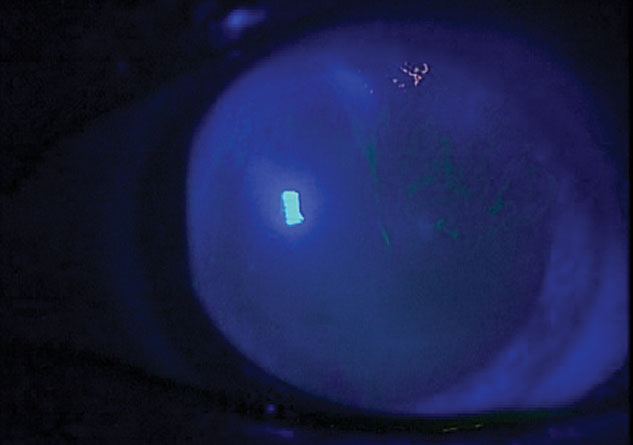
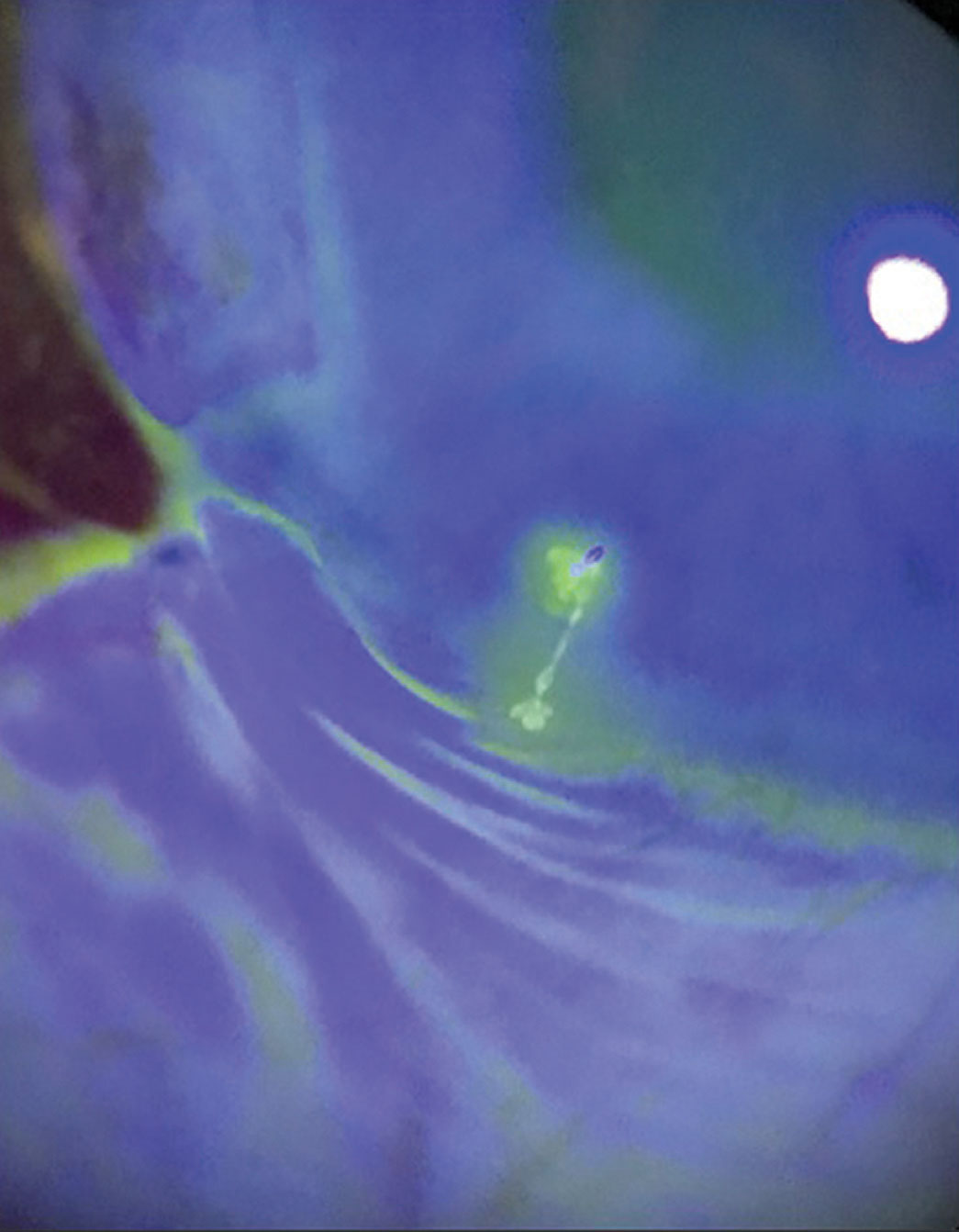 |
This Sjögren’s syndrome patient has filamentary keratitis. |
Who’s Hung Out to Dry
Some of the common systemic diseases associated with dry eye ODs may encounter in clinical practice include:
Diabetes. The estimated 30.2 million Americans (12.2% of the population) with diabetes mellitus (DM) type 1 or 2—and another estimated 84.1 million who are prediabetic—are all more prone to DED and ocular surface disease.3,4 A recent study found that 51.3% of the participants with diabetes had DED but were undiagnosed.5 These patients often have a decreased tear break-up time (TBUT) and tear instability related to the decreased mucin layer caused by a reduction in goblet cell density in the conjunctiva.1,4 When testing diabetes patients for DED, clinicians should be aware that not all tests are equal. One study compared diagnostic tests in this patient population and found that the Ocular Surface Disease Index (OSDI) only detected 17.1% of DED patients, which was the lowest compared with TBUT, Schirmer’s score, corneal/conjunctival staining and tear osmolarity.6
MGD is also a major cause of DED in patients with type 2 diabetes. A study of diabetic patients and DED patients without DM looked at the OSDI score, TBUT, Schirmer’s score, corneal staining, lipid layer thickness, meibomian gland parameters, HbA1C and duration of diabetes.7 The researchers found tear volume in DED patients without DM was higher than in patients with DM; however, the meibomian gland parameters were higher with normal OSDI scores in the diabetic patients without DED.7 Thus, MGD likely presents before ocular symptoms in this population, and clinicians should treat any signs of DED, regardless of whether DM patients are complaining of symptoms.7
Research also suggests these patients may experience damage to the microvascular supply of the lacrimal gland, causing decreased production and reduced lacrimal innervation from the autonomic neuropathy and decreased conjunctival injection.1,5,8 Notably, a decrease in tear secretion may be more severe in patients with non-proliferative diabetic retinopathy (NPDR) compared with those without NPDR.4,9
In conjunction, patients with diabetes experience a decrease in corneal sensitivity and wound healing known to lead to, among other problems, a reduced reflex and basal tear secretion.5 These patients are at a higher risk for DED progression and non-compliance with treatment because they don’t note early dry eye symptoms.
The recommended yearly diabetic eye exam to monitor for ocular signs of complications should include a careful assessment of the ocular surface for signs of dry eye.6,10 At the very least, this ought to involve TBUT and both corneal and conjunctival staining. Clinicians should also consider adding tear osmolarity and meibography.6
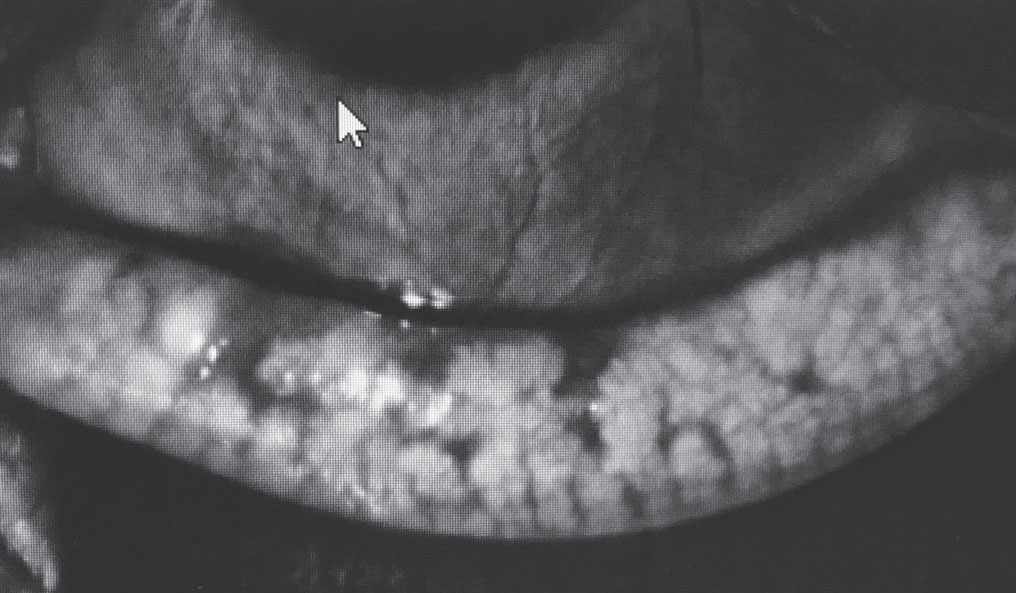 |
In this meibography of a diabetic patient with MGD, note the gland truncation and poor gland structure. |
Chronic inflammatory diseases. These conditions, including rheumatoid arthritis (RA), Sjögren’s syndrome (SS), inflammatory bowel disease (IBD) and sarcoidosis can all contribute to an increase in DED. One study found that 11% of RA patients had persistent ocular and oral dryness, and 17.5% had sporadic dryness—a 1.24x increased risk compared with patients not diagnosed with RA.11 The prevalence of ocular dryness also increased by 10% to 13% for every 10 years of treatment.11 Higher rates of self-reported body pain and fatigue were also noted to have the highest clinical correlation to dryness.11 A study in the United Kingdom also found that 70% of RA patients had dry eye, but only 12% were being treated for it.12
SS is most typically identified by the altered lacrimal and salivary gland function and may be present in conjunction with other autoimmune syndromes such as RA, Wegener’s granulomatosis and systemic lupus erythematosus (SLE).13,14 One study found 57% of patients with SLE also suffered from pathological dry eye.15
With SS, the exocrine glands are infiltrated by lymphocytes CD4+ T- and B-cells causing dysfunction and destruction.16 The most commonly affected are the salivary and lacrimal glands, which can be biopsied to confirm diagnosis. Research shows increased expression of cell surface adhesion molecule ICAM-1, which binds to LFA-1 on lymphocytes and recruits antigen-presenting cells that initiate an immune-mediated inflammatory response.16 This chronic inflammation leads to worsening of DED and keratoconjunctivita sicca, which can be monitored with corneal/conjunctival staining, TBUT and tear osmolarity.16
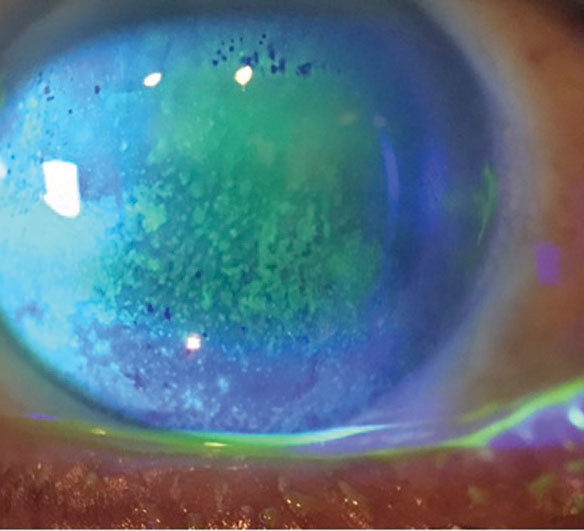 |
| This DED patient on medication for depression has diffuse superficial punctate keratitis. |
The inflammatory disease sarcoidosis causes collections of granulomas to form within organs throughout the body. Most commonly, these non-caseating granulomas form in the lungs, lymph nodes, lacrimal glands and skin.17 Approximately 40% of sarcoidosis patients have ocular manifestations, with uveitis being the most common, but exocrine gland and lacrimal gland involvement can occur.17,18 When the exocrine gland is affected, it can cause symptoms of both dry eye and mouth similar to SS.17,19
Because sarcoidosis and SS present similarly, bloodwork and a biopsy are often necessary to differentiate the two. When the lacrimal gland develops granulomas, it can cause ocular adnexa swelling and a decrease in lacrimal production, leading to both aqueous deficiency and mechanical exposure from potential lagophthalmos.17-19
Patients with IBD often experience ocular manifestations such as episcleritis, scleritis, iritis and DED.20,21 One study found that 22% of patients with IBD experienced DED, in comparison with only 11% of the control patients.20 Pathophysiology of this increased diagnosis of DED in IBD patients is poorly understood, but researchers suspect an increased inflammation due to local action of antigen-antibody complexes that are produced against the bowel wall vessels and transported via the blood stream.21
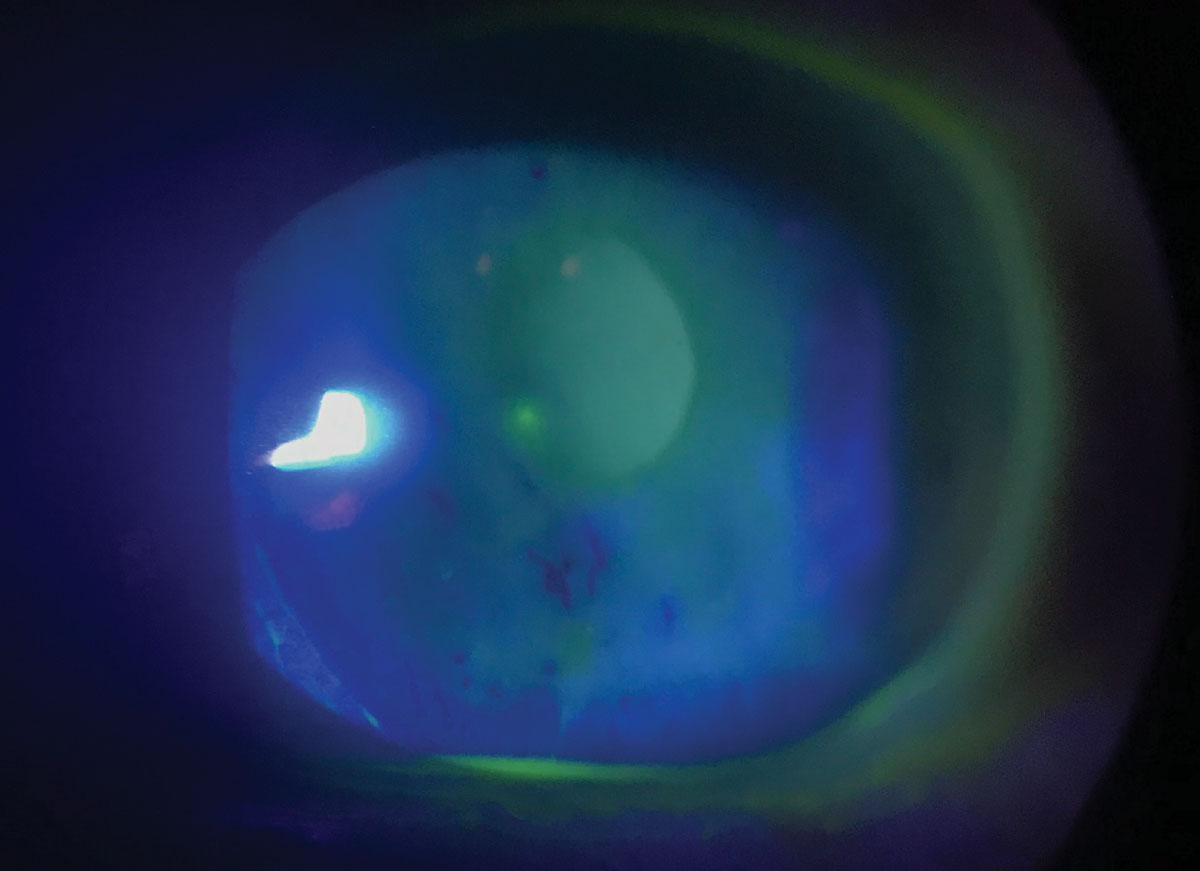 |
Dry eye is a common ocular side effect of both hyper- and hypothyroidism. Photo by Matt Dixon, OD. |
Thyroid dysfunction. Graves’ orbitopathy, or thyroid eye disease, is an autoimmune disease where the patient’s thyroid-stimulating hormone receptor auto-antibodies cause excess thyroid hormone production. It is most frequently seen in patients with hyperthyroidism but can also be found with hypothyroidism and euthyroid states.22 This condition causes orbital tissue inflammation, leaving these patients more prone to ocular surface problems and DED. One study estimates as many as 85% of Graves’ orbitopathy patients experience dry eye symptoms.22
Physical changes such as lagophthalmos and exophthalmos can lead to poor lid closure, causing dryness from exposure and incomplete blinking.22 This may also result in high tear osmolarity secondary to evaporation.22 Researchers suspect the reduction in tear production occurs due to the inflammatory process that causes lacrimal gland deficiency.22 These physical changes may also be an indicator in patients who are being treated for dry eye that a thyroid issue may be present and warrant bloodwork, including T3, T4, TSH and TSI.
These patients often experience an active phase of Graves’ orbitopathy within 12 to 18 months of the manifestation of systemic symptoms and then go into remission, with the possibility of reactivation in the future.22 Once out of the active phase, symptoms of Graves’ orbitopathy may lessen or even resolve, depending on severity.22
Working with the patient’s physician is important for systemic treatment to decrease ocular effects of thyroid imbalance.
Migraines: Double-edged SwordsUnfortunately for patients known to suffer from migraines, DED can both exacerbate the condition and be caused by it at the same time.1 A study found that patients who experience migraines had decreased mean TBUT and Schirmer’s scores and increased OSDI scores and lissamine green staining when compared with the control group.1 These patients may also be more likely to experience dry eye because of corneal physiological changes. One study used confocal microscopy to view the sub-basal corneal nerve plexus in chronic migraine patients. Compared with non-migraine sufferers, these patients had significantly decreased nerve fiber density, decreased nerve fiber length and symptoms of DED.2 1. Koktekir BE, Celik G, Karalezli A, Kal A. Dry eyes and migraines: is there really a correlation? Cornea. 2012;31(12):1414-6. 2. Kinard KI, Smith AG, Singleton JR, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache. 2015;55(4):543-9. |
Dermatological conditions. Acne rosacea and acne vulgaris can manifest with ocular sequelae, including eye dryness. In the case of rosacea, this chronic skin disorder affects the sebaceous glands, and ocular involvement can include irritation, blepharitis, epiphora and eyelid margin erythema telangiectasia.23 Ocular findings can occur with or without the primary skin findings, and the estimated incidence varies between 6% and 72%.23 The mechanism isn’t well understood, but chronic inflammation is at the root of ocular symptoms.23 Inflammation of the meibomian glands can cause abnormal lipid secretion and clogged glands, leading to tear film quality changes clinicians can measure with both TBUT and tear osmolarity.23
Dry eye in patients with acne vulgaris is usually related to the medication rather than the disease. Isotretinoin, which is commonly used to treat acne vulgaris, causes decreased density and atrophy of the meibomian glands, leading to ocular surface problems.24 One study noted a decreased TBUT in 69.1% of patients and the development of blepharitis in 40% of patients.25 Another study found similar results of decreased TBUT in 50% of patients and blepharitis in 55%.26 In both studies, the changes disappeared within one month of patients discontinuing the medication.25,26 However, when a patient experiences permanent sebaceous gland dysfunction and meibomian gland dropout, the DED will not likely resolve with discontinuation of the medication.26
Psychological conditions. For patients with anxiety or depression, DED can be exacerbated by both the medication and physiological changes. Research shows chronic depression can worsen DED because of an increase in pro-inflammatory cytokines production.27 It can also hinder the patient’s ability to deal with the discomfort and pain of the condition, as well as feed into a negative feedback loop that exacerbates the depression or anxiety.27 Unresponsive dry eye patients who also exhibit depressive behavior can be referred for an evaluation with their primary care provider if not already diagnosed and under treatment.
A large Veterans Affairs study found patients who experienced DED had a higher odds ratio for both post-traumatic stress disorder (PTSD) and depression.27,28 The data showed that 19% of male patients and 22% of female patients diagnosed with PTSD were also diagnosed with DED. An odds ratio of 1.92 DED risk was calculated for PTSD patients. An increased risk was found for the same patients who were using multiple systemic medications.28
Attention deficit hyperactive disorder (ADHD) has been linked to an increased incidence of DED, and the medications used to treat ADHD, Adderall (dextroamphetamine/amphetamine, Teva Pharmaceuticals) and Ritalin (methylphenidate hydrochloride, Novartis), can also cause dry eye.29 One study found patients with ADHD had a higher OSDI score and rate of DED symptoms compared with non-ADHD patients.29 However, limited information exists and the mechanism is poorly understood, indicating further studies are necessary to further investigate the validity of this claim.
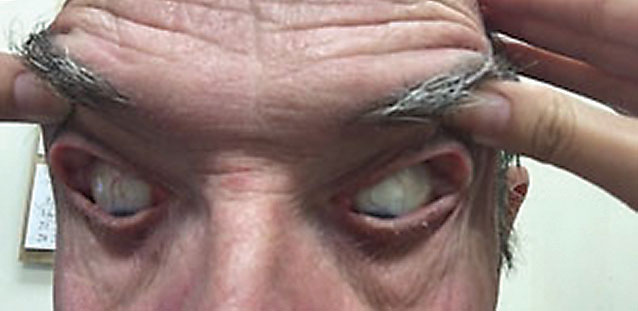
 |
This patient with OSA has DED and anterior membrane dystrophy. |
Parkinson’s disease (PD). These patients are predisposed to dry eye caused by both motor function and physiological changes. Blink rate is significantly decreased, allowing for more exposure time of the cornea and an increase in dryness. One study found PD patients had an average blink rate of 12.7 times per minute, significantly lower than the control patients’ average blink rate of 21.8 times per minute.30 The same study noted that PD patients had significantly decreased meibomian gland function and tear meniscus height.30 Another study found lower Schirmer scores and higher overall OSDI scores.31 Researchers speculate this is due to decreased androgen levels and autonomic dysfunction caused by Lewy bodies at the sympathetic ganglia, substantia nigra and peripheral parasympathetic ganglia.32,33
Also, researchers believe oxidative stress occurs in both Parkinson’s and Alzheimer’s disease, further exacerbating DED.19 Mice studies found that the functional knockout of the mev-1 gene caused a decrease in tear production, lacrimal dysfunction and DED and conclude that oxidative stress causes inflammation and leads to these problems.34,35
Future studies with human subjects may one day show the same correlation, providing an earlier indicator of DED and the need for early intervention for those who suffer from diseases with known oxidative stress.
 |
This patient’s elastic, easily pliant eyelids prompt a diagnosis of FES, which is linked to several severe ocular and systemic diseases. Photo by Victoria Roan, OD. |
Neuropathic pain. This is caused by a lesion or disease of the somatosensory system, leading to sensitization of the peripheral and central nerves. In turn, this causes maladaptive neuroplastic changes to both the PNS and CNS sensory processing pathways. A discrepancy in signs and symptoms can be found in a subgroup of DED. These patients also show signs of ocular neuropathic pain, including abnormal sensations, spontaneous pain, pain from light or wind and exaggerated pain to normal stimulus.27
Ocular neuropathic pain can happen with reoccurring corneal nerve injury or inflammation, which causes chronic changes to both the peripheral and central corneal somatosensory pathways. Research has found this same mechanism of corneal nerve injury and regeneration in LASIK patients, which is believed to be the cause of dry eye symptoms post-surgery.27
Studies have also shown chronic pain syndrome (CPS) is associated with DED. One study of 154 patients found that patients with CPS had worse dry eye symptoms and ocular pain compared with controls.27 These patients may also be more prone to neuropathic ocular pain, which would exhibit in the clinic as pain out of proportion with ocular signs of DED.27
 |
Sleep disorders. The lack of quality sleep—the right amount of REM cycles, or deep sleep—can lead to myriad adverse outcomes, including circadian rhythm disruption, hypertension, metabolic syndrome and even ocular sequelae.36-38 A recent study of the association between sleep quality and DED found 45% of DED patients also reported poor sleep quality.36 Prior studies found tear instability and decreased secretion related to changes in circadian rhythm, as well as lower tear secretion in metabolic syndrome.36,38,39
Nearly 18 million adults in America suffer from obstructive sleep apnea (OSA), according to the National Sleep Foundation.40 This condition has also been linked to floppy eyelid syndrome (FES), corneal erosions, keratitis and punctate corneal epitheliopathy.41 These patients often experience a continual state of inflammation in the eye due to increased accumulation of pro-inflammatory cytokines produced by the lacrimal gland caused by chronic intermittent hypoxia. This in turn damages the meibomian glands and goblet cells, decreases corneal sensitivity and reduces tear production.41,42
Studies looking at DED in patients with OSA without FES found higher OSDI scores and significantly decreased TBUT and Schirmer scores in moderate and severe sleep apnea patients when compared with those in the control group.41 Dryness can also be exacerbated by ill-fitting continuous positive airway pressure masks that leak air toward the eye.
FES is caused by lid laxity due to a decrease in elastin content, lending the patient to spontaneous inversion, eversion or both.43 The spontaneous nocturnal eyelid eversion leads to irritation of the conjunctiva and possible corneal abrasions. Poor apposition of the lids causes a compromise of the tear film, in theory leading to DED.43
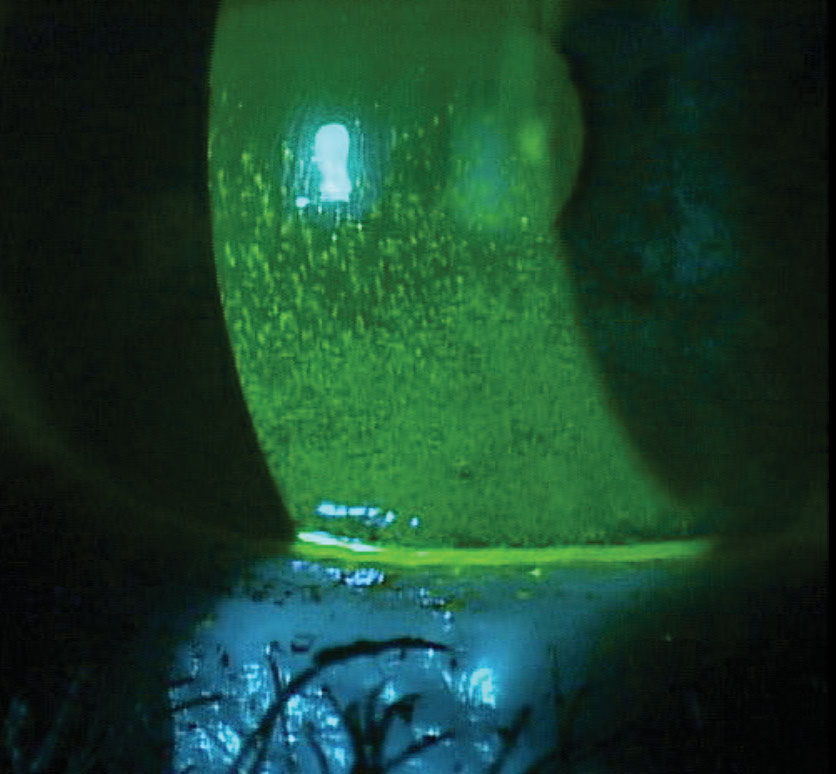 |
| Significant corneal staining in a patient with Sjögren’s, fibromyalgia and rheumatoid arthritis. Photo by Mile Brujic, OD, and Jason Miller, OD, MBA. |
Although not all patients with OSA have FES, an increased incidence exists in patients with OSA. Those with both conditions have a higher correlation of DED than patients with OSA only.41
Medication Woes
Many pharmaceuticals used to treat psychological conditions, dermatological conditions, allergies, epilepsy and seizure disorders can cause or exacerbate DED (Table 1). When discussing a patient’s systemic disorder and related medications, it is important to be thorough, as many patients may be resistant to discussing their health history without understanding the connection with their ocular health.
Often, discontinuing an offending medication is not an option. In these cases, optometrists must address the patient’s ocular signs and symptoms as best as possible. Clinicians should always comanage with the treating physician to discuss alternatives when DED therapy isn’t working and the patient’s vision is at risk.
While all patients should be treated as a whole, those with DED may warrant a little extra care. Many systemic conditions can cause or exacerbate dry eye, as can the various treatments necessary to maintain a patient’s quality of life.
Optometrist should remain familiar with ongoing research to help uncover more concrete evidence of these associations, as many studies have yet to discern the true cause of DED—the systemic condition or the systemic medication. Because neither one is often modifiable, ODs must be prepared for the ongoing care and comanagement these patients need.
The first step is identifying the comorbidities themselves. Only then can clinicians make informed treatment decisions to improve patient comfort and satisfaction.
Dr. Koetting is the referral optometric care and externship program coordinator at Virginia Eye Consultants in Norfolk, VA. She is a fellow of the American Academy of Optometry and a trustee of the Virginia Optometric Association.
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-65. 2. Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15(3):284-333. 3. Center for Disease Control. National Diabetes Statistics Report, 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. July 18, 2017. Accessed August 20, 2018. 4. Skarbez K, Priestley Y, Huepf M, Koevary SB. Comprehensive review of the effects of diabetes on ocular health. Expert Rev Ophthalmol. 2010;5:557-77. 5. Schwartz S, Halleran C, Doll T, et al. Does diabetes make a difference in dry eye? ARVO 2018. Abstract 956-B0134. 6. Najafi L, Malek M, Valojerdi AE, et al. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: a diagnostic accuracy study using STARD standard. J Diabetes Metab Disord. 2015;14:39. 7. Wu H, Xie F, Luo S, et al. Meibomian gland dysfunction is an early sign and major cause of dry eye in Type 2 diabetes. ARVO 2018. Abstract 4900-C0345. 8. Kaiserman I, Kaiserman N, Nakar A, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. 2005;139:498-503. 9. Yoon KC, Im SK, Seo MA. Changes in tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18:168-74. 10. American Optometric Association. Evidence-based clinical practice guideline: Eye care of the patient with diabetes mellitus. 2014. 11. Wolfe F, Kaleb M. Prevalence, risk, and risk factors for oral and ocular dryness with particular emphasis on rheumatoid arthritis. J Rheumatol. 2008;35(6):1023-30. 12. Piper H, Douglas KM, Treharne GJ, et al. Prevalence and predictors of ocular manifestations of RA: is there a need for routine screening? Musculoskeletal Care. 2007;5(2):102-17. 13. Gilboe IM, Kvien T, Uhlig T, Husby G. Sicca symptoms and secondary Sjögren’s syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheumatic Dis. 2001;60(12):1103-9. 14. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 15. Ostanek L. Ocular manifestations in patients with systemic lupus erythematosus and antiphospholipid syndrome. Pol Arch Med Wewn. 2007;117 Suppl:18-23. 16. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609-14. 17. Drosos AA, Constantopoulos SH, Psychos D, et al. The forgotten cause of sicca complex; sarcoidosis. J Rheumatol. 1989;16(12):1548-51. 18. Groen F, Rothova A. Ocular involvement in sarcoidosis. Semin Respir Crit Care Med. 2017;38(4):514-22. 19. Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82(5):885-98. 20. Felekis T, Katsanos K, Kitsanou M, et al. Spectrum and frequency of ophthalmologic manifestations in patients with inflammatory bowel disease: a prospective single center study. Inflamm Bowel Dis. 2009;15(1):29-34. 21. Troncoso LL, Biancardi AL, de Moraes HV Jr, Zaltman C. Ophthalmic manifestations in patients with inflammatory bowel disease: a review. World J Gastroenterol. 2017;23(32):5836-48. 22. Selter JH, Gire AI, Sikder S. The relationship between Graves’ ophthalmopathy and dry eye syndrome. Clin Ophthalmol. 2015;9:57-62. 23. Arman A, Demirseren DD, Takmaz T. Treatment of ocular rosacea: comparative study of topical cyclosporine and oral doxycycline. Intern J Ophthalmol. 2015;8(3):544-9. 24. Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10(4):286-90. 25. Egger SF, Huber-Spitzy V, Böhler K, et al. Ocular side effects associated with 13‐cis‐retinoic acid therapy for acne vulgaris: clinical features, alterations of tearfilm and conjunctival flora. Acta Ophthalmologica Scandinavica. 1995;73(4):355-7. 26. Bozkurt B, Irkec MT, Atakan N, et al. Lacrimal function and ocular complications in patients treated with systemic isotretinoin. Eur J Ophthalmol. 2002;12:3:173-6. 27. Han SB, Yang HK, Hyon JY, Wee WR. Association of dry eye disease with psychiatric or neurological disorders in elderly patients. Clin Interv Aging. 2017;12:785-92. 28. Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012;154(2):340-6.e2. 29. Cho KJ, Kim HK, Lim MH, et al. Depression, ADHD, job stress and sleep problems with dry eye disease in Korea. Am J Psychiatry. 2015;18:331. 30. Cengaver T, Melek IM, Duman T, Oksüz H. Tear film tests in Parkinson’s disease patients. Ophthalmology. 2005;112(10):1795. 31. Nowacka B, Lubinski W, Honczarenko K, et al. Ophthalmological features of Parkinson disease. Med Sci Monit. 2014 Nov;20:2243-9. 32. Kandel ER, Schwartz, Jessell TM. Principles of Neural Science. 3rd ed. New York: Elsevier; 1991. 33. Okun MS, McDonald WM, DeLong MR. Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol. 2002;59:807-11. 34. Uchino Y, Kawakita T, Miyazawa M, et al. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS One. 2012;7(10):e45805. 35. Yi W, Asbell PA. The core mechanism of dry eye disease (DED) is inflammation. Eye Contact Lens. 2014;40(4):248-56. 36. Kawashima M, Uchino M, Yokoi N, et al. The association of sleep quality with dry eye disease: the Osaka study. Clin Ophthalmol. 2016 Jun 1;10:1015-21. 37. Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10(Suppl 2):37-45. 38. Wolk R, Somers VK. Sleep and the metabolic syndrome. Exp Physiol. 2007;92:67-78. 39. Yaguchi S, Ogawa Y, Shimmura S, et al. Angiotensin II type 1 receptor antagonist attenuates lacrimal gland, lung, and liver fibrosis in a murine model of chronic graft-versus-host disease. PLoS One. 2013;8:e64724. 40. Hauri P. The Sleep Disorders. National Sleep Foundation. 2018. 41. Karaca EE, Akçam HT, Uzun F, et al. Evaluation of ocular surface health in patients with obstructive sleep apnea syndrome. Turk J Ophthalmol. 2016;46(3):104-8. 42. Acar M, Firat H, Acar U, Ardic S. Ocular surface assessment in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2013;17:583-8. 43. Miyamoto C, Santo LCE, Roisman L, Osaki M. Floppy eyelid syndrome. Arquivos Brasileiros de Oftalmologia. 2011;74(1):64-6. |
