 |
Demystifying the Complement System
Understanding the range of structural findings associated with this condition is key to effective management.
By Anna Bedwell, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: February 15, 2024
Expiration Date: February 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists who want to learn about the impact this system has on practice.
Educational Objectives: After completing this activity, participants should be better able to:
Comprehend the physiology and pathophysiology of the complement cascade.
Recognize the complement system’s involvement in AMD.
Identify current treatment options that target the complement system.
Effectively manage patients with geographic atrophy.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Bedwell is a consultant for Iveric Bio. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #127640 and course ID 89405-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
The onset of new geographic atrophy (GA) treatments, both targeting complement inhibition, has sparked renewed interest in the complement pathway. Eyecare providers understand VEGF and its role in exudative macular degeneration; however, do they similarly understand the complement system, as well as its relationship with the other advanced form of AMD? Probably not, as most of us haven’t thought much about the complement pathway since optometry school.
This system is undoubtedly complex, but this article will break it down into a simpler understanding focusing on its relationship with age-related macular degeneration (AMD) and pertaining to GA treatments that have recently become available.
 |
|
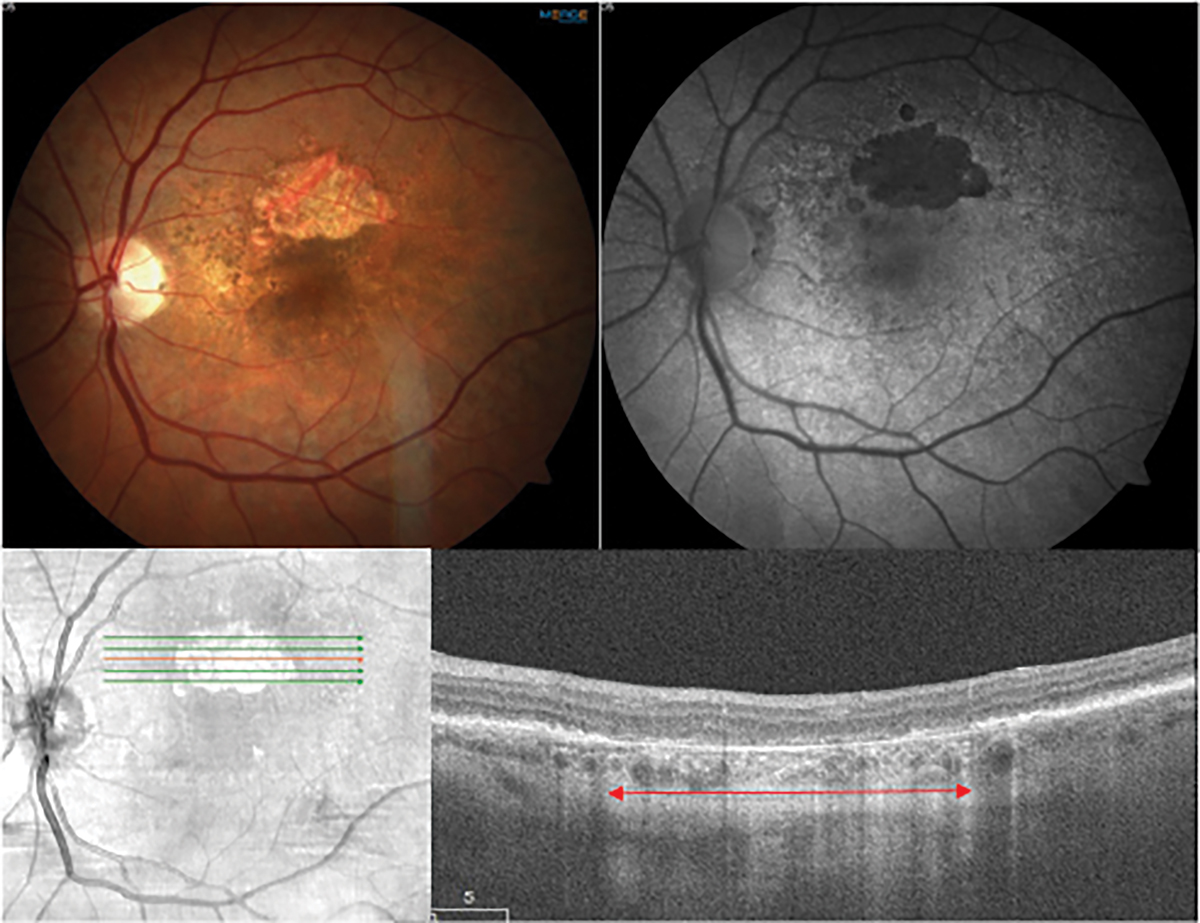
Fig. 1. Multimodal imaging of geographic atrophy. The top row shows a color fundus photo of a non-foveal island of GA in dry AMD and a fundus autofluorescence image in which the atrophy demonstrates classic hypoautofluorescence. The bottom row shows a near infrared image and an OCT scan through the GA lesion. There is a loss of ellipsoid zone and RPE in the outer retina and a choroidal hypertransmission defect (red arrow). Click image to enlarge. |
The Complement System
A cascade of proteins (more than about 50) in membrane-bound and fluid-phase, the complement system is responsible for removing cellular debris and pathogens like bacteria.1 It does this by using opsonization (a form of tagging), attracting phagocytes via chemotaxis and targeting cells for lysis.1 Tissue homeostasis relies on the complement pathway to continuously turn over. The complement system operates locally in the eye as well as systemically, produced in the liver. In the retina, the complement system maintains the retinal integrity by several means, including clearing the turnover of photoreceptor outer segments in the retinal pigment epithelium (RPE).2
The complement system must sustain tight regulation to avoid unwarranted inflammation and subsequent tissue damage. Overactivation of the pathway promotes the pathogenesis of systemic disease and organ-specific disease such as AMD. Individuals with AMD have been found to have increased levels of complement end product in the choriocapillaris.3,4 Additionally, complement components have been found in the retina, particularly in drusen.3,5 A basic understanding of the complement system helps to understand this relationship with AMD and why the system has been targeted as a means of GA treatment.
Let’s break down the complement cascade into its four main sections: the pathways, C3 convertase, C5 convertase and formation of the membrane attack complex (MAC).
1. The pathways. Three pathways mark the start of the cascade: classical, lectin and alternative. Each pathway operates independent of one another but ultimately converges toward a final result in the MAC, responsible for creating a pore on pathogens and debris, resulting in cell lysis.
The classical pathway is dependent on an adaptive immune response, meaning the pathogen must have been previously combated to create an antibody response.1 Antibodies, such as IgG and IgM serve as activators.1 This pathway is triggered when antibodies bind to the protein C1q, which in turn recognizes and binds to the surface of an invading pathogen. This forms an antibody-antigen complex, leading to the split of two proteins, C2 and C4. The fragments of these proteins, C2a and C4b, join together to form the enzyme C3 convertase (C4b2a).
The lectin pathway operates similar to the classical except that it isn’t antibody driven. Rather, mannose-binding lectin and its binding partners, mannose-associated serine proteases, recognize and bind to the carbohydrate structures (oligosaccharides) found on a microbial surface to form a complex. This complex drives the split of C2 and C4, akin to the classical pathway, to ultimately result in the same C3 convertase.
The dominant player in the complement system, the alternative pathway, is credited for over 80% of complement system end product of MAC formation.6 The alternative pathway is constantly in action at low levels via spontaneous hydrolysis of C3, also known as complement “tickover,” making it ready for immediate C3b deposition on pathogens for target opsonization, the process of tagging pathogens for phagocytic lysis.
In a multistep process, spontaneous cleavage of the thioester bond in C3 creates a distinct C3 convertase (C3bBb). It binds with properdin, a serum protein, to stabilize. Then, this distinct form starts an amplification loop cleaving C3 into C3a and C3b. The C3b is in turn used to create extra C3 convertase, keeping the terminal pathway going to form MAC. This process is regulated by complement factors, the enzyme mediators of the system. These regulators help to focus the complement activity specifically to pathogens. The complement factors will be described in a later section when it applies to genetic risk for AMD development.
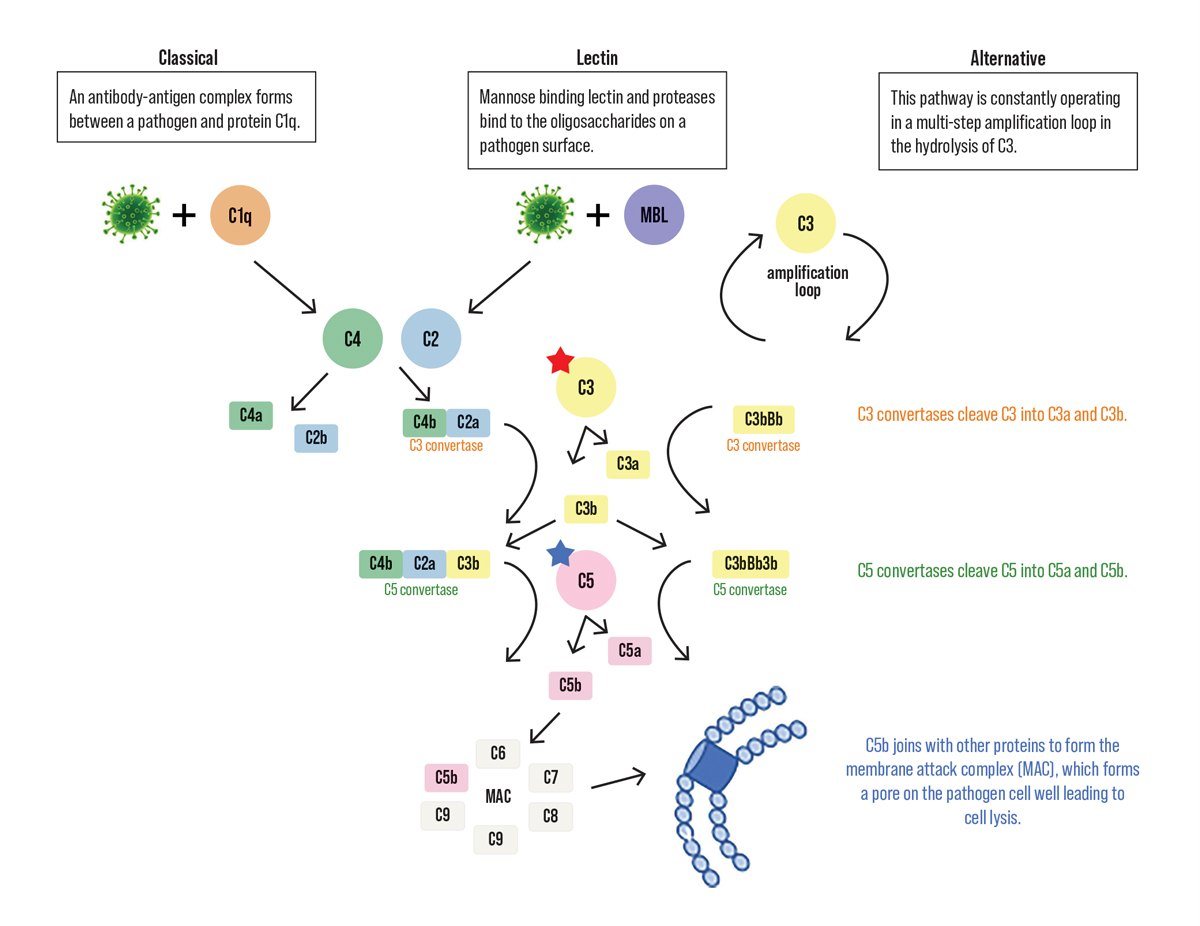 |
| Fig. 2. Simplified schematic of the complement pathway from the pathways to the terminal endpoint of the membrane attack complex. The inhibition target for pegcetacoplan is C3 indicated by the red star. The blue star highlights C5, the inhibition target for avacincaptad pegol. Click image to enlarge. |
2. C3 convertase. The three pathways converge at C3 convertase, an enzyme family. The C3 convertase produced via the classical and lectin pathways is C4b2a while the alternative results in C3bBb. Both of these C3 convertases operate the same by cleaving C3 into C3a and C3b. C3a is an anaphylatoxin, a molecule that prompts chemotaxis, the movement of eosinophils and phagocytic cells to generate increasing concentrations of C3a. C3b keeps the complement pathway moving. It is an unstable molecule that quickly stabilizes by forming a covalent bond to the surface of microbial cells triggering phagocytosis.
3. C5 convertase. The enzymes C3 and C5 convertase differ in their C3b requirement. C5 convertase necessitates at least two molecules of C3b to form. In the previous step, extra C3b molecules were generated. These bind to a C3 convertase and in turn become C5 convertase. The role of C5 convertase is to cleave C5 into C5a and C5b. C5a is another anaphylatoxin like C3a described above. C5b then initiates the terminal pathway described next.
4. Membrane attack complex (MAC). The terminal step of the complement system is the assembly of MAC, a group of five globular plasma proteins. The C5b generated in the preceding step combines with C6, C7, C8 and multiple C9s. This complex attaches to the cell membrane and forms a pore, destroying the membrane integrity and resulting in cell death.
Genetic Associations
In 2001, one study discovered complement byproducts in drusen, most notably complement factor H (CFH), implying the complement system’s involvement in AMD.7 To date, multiple genome-wide association studies have confirmed at least 52 independent gene variants and 34 genetic loci, accounting for over half of the genetic risk for AMD.8 A portion of these identified high-risk genetic variants code for key enzymes and proteins of the complement system.8,9
However, the complement system is not the only contributor, as other variants are tied to extracellular matrix remodeling, lipid metabolism, angiogenesis and oxidative stress response demonstrating the complex AMD pathophysiology. These genetic variants span from common in the population, typically conveying a low AMD risk, while other variants are quite rare but impart a nearly complete penetrance for AMD development. About two-thirds of the heritability of AMD is attributed to the common variants, the remaining attributed to rare variants which are more likely to be disease causative.10 The variants are referred to as single-nucleotide polymorphisms (SNPs), genomic variants at a single base position in the DNA.
Complement factors. Of the three initial pathways, the alternative pathway has been linked most frequently with AMD development.4 Complement factors B, D, F, H and I within the alternative pathway all have genetic variants discovered to be associated with AMD.11-13 The most well-established of these is the CFH gene found on chromosome 1.7 In the complement pathway, it regulates the alternative pathway by acting as a cofactor to complement factor I (CFI) in the inactivation of C3b. When CFH function is compromised, dysregulation of the complement cascade occurs, allowing for damage to healthy tissue rather than isolating to pathogens. CFH risk variants have a slightly greater association with risk for GA vs. exudative AMD.8,14
A substantial portion of the AMD genetic risk lies in two CFH variants: rs1061170 (also known as Y402H) and rs1410996. Thirty percent of the population of European descent carry at least one copy of the Y402H allele.15 Those with one allele have a 2.3-fold increased risk of AMD, which jumps to a 5.2-fold risk with two alleles.15 Other, less common CFH variants have also been discovered, some of which are associated with an exceedingly high risk for advanced AMD, such as R1210C. This variant has been linked to a much younger onset age of AMD and displays the strongest genetic risk for AMD of variants found to date.10
CFI, mentioned earlier as cofactor to CFH in the alternative pathway, also has genetic variants linked to AMD. In particular, it holds several rare variants, with at least 59 identified.12 One study found that 7.8% of AMD cases vs. 2.3% of controls harbored rare missense CFI variants.11 These rare variants were associated with a 7.5-fold elevated risk of advanced AMD.11 CFI is a rate-limiting enzyme of complement termination. In vitro study has found that increasing CFI concentration by just 25% can essentially shut down alternative pathway activation.16,17
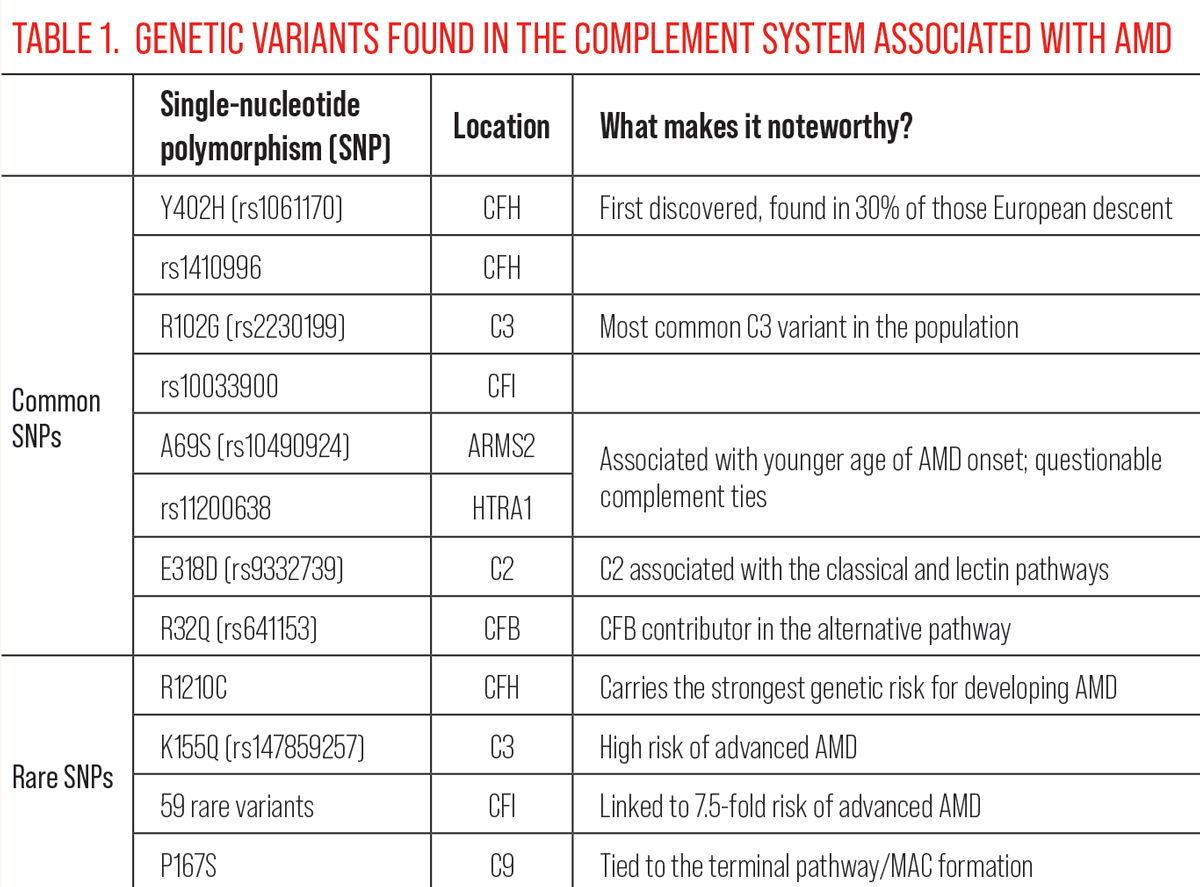 |
| Click table to enlarge. |
Complement components. Several AMD-associated variants have been found in the complement C3 gene. The three complement pathways converge on C3, making it a critical step of the complement system. The most common variant in the population associated with C3 is R102G.18,19 Another variant of interest is K155Q, which results in feedback amplification of the alternative pathway with an effect very similar to the aforementioned R1210C variant in CFH.11
Complement component 9 (C9) also shows ties to AMD, particularly in P167S.11 The previously mentioned variants all were associated with the alternative pathway. However, C9 is responsible for the formation of the MAC in the terminal pathway, reminding us that there is not a single route by which the complement system contributes to AMD development.
Another well-established genetic locus in ARMS2-HTRA1 may have ties to the complement system, but this remains unclear. Multiple high-risk variants have been found in the ARMS2-HTRA1 region of chromosome 10. Together, these variants in combination with CFH variants account for over half of the genetic risk associated with AMD.8,20,21 The mechanisms of AMD development from dysfunction in the ARMS2-HTRA1 region is not well understood. At least in part, ARMS2 appears to be involved in complement-mediated clearance of cellular debris.22 As such, protein deficiencies in ARMS2 may be involved in drusen formation.22
Genetic testing in AMD. With the genetic ties mentioned, will genetic testing become a standard of care for AMD? Perhaps. It has certainly become more commonplace to use genetic screening for assessing risk and considering suitability for vitamin supplementation. Presently, the caveat lies in that AMD is a multifactorial disease involving genes and environmental factors—such as smoking, nutrition and body mass index—all playing a role. This makes risk stratification tricky. There are two commercially available AMD genetic testing providers: Arctic Medical Laboratories and Visible Genomics. Arctic tests for 14 SNPs associated with AMD and Visible Genomics tests for 15 SNPs. Both use an algorithm based on genetic and environmental risk factors.
Visible Genomics offers two tests: one that assesses the risk of developing AMD and the other for those with early or intermediate stage AMD to assess risk for advanced stage disease. On the other hand, Arctic only offers genetic tests for those who already have a diagnosis of drusen or any stage of AMD. They offer two types of tests: the Macula Risk test, which gives a two-, five- and 10-year risk for progression to advanced AMD and Vita Risk, which can be ordered stand-alone or added to Macula Risk, which uses the patient’s genetic profile to guide whether AREDS formulation, antioxidants alone or zinc alone would be most beneficial. This is based on research—albeit controversial—finding that high-dose zinc can increase risk of advanced AMD in those with CFH risk alleles.23-25
Genetic testing in AMD remains a debated topic. Those that argue for it find that patients can benefit from more frequent follow-up for early detection of progression to advanced stage and an opportunity to tailor preventive nutritional supplements. The American Academy of Ophthalmology does not recommend genetic testing for AMD.26 However, if a gene-targeted treatment were to be approved, then genetic testing would quickly become a necessity.
Treatment Targeting Complement
The genetic and biological evidence that complement is a major driver in AMD has been established. However, targeting the complement system with treatment isn’t as straightforward. There are many considerations, given that the ideal target treatment point within the complement cascade remains debated. It is also important to note that the complement system has many necessary functions in preventing infection. Can that just be turned off?
Historically, previous treatments aimed at the complement system, such as eculizumab (targeting C5) and lampalizumab (targeting CFD), failed.27 However, the treatment landscape for GA changed completely in 2023 with the FDA approval of two complement inhibitors for the treatment of GA secondary to AMD. Below we will discuss how these treatments fit into the complement puzzle.
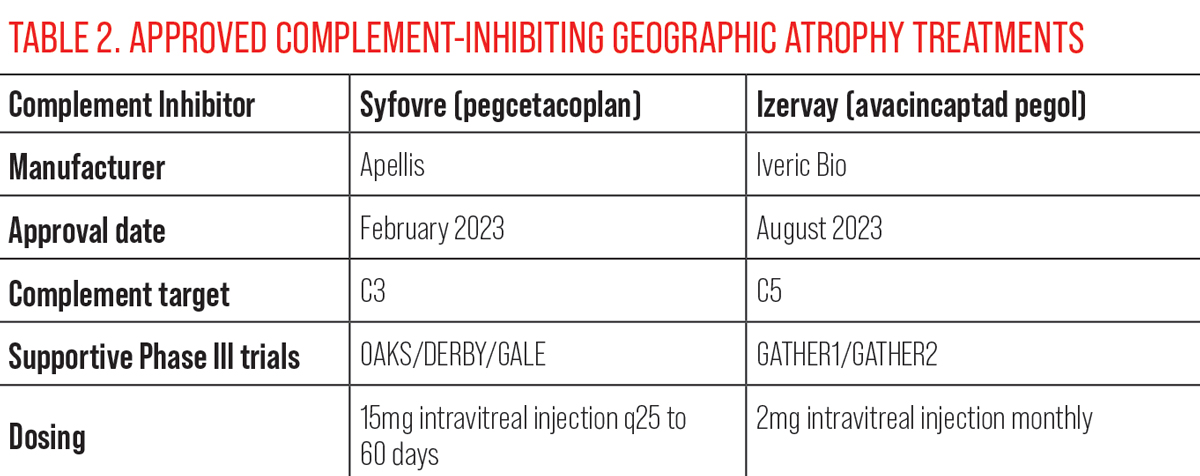 |
| Click table to enlarge. |
The first approval came in February with Syfovre (pegcetacoplan injection), a C3 inhibitor.28 Syfovre is composed of two cyclic peptides attached to a polyethylene glycol chain, to provide a longer half-life and enhance solubility.29 The peptides bind to C3 and the activation fragment C3b to halt production of the complement system.29 The approval came at the heels of two Phase III studies: DERBY and OAKS.
At 12 months, OAKS showed a significant reduction in GA expansion, whereas DERBY did not.30 This improved for both trials at the 24-month follow-up in monthly and every-other-month treatment groups.31 GALE, a phase III extension study from DERBY and OAKS, evaluating the 36-month long-term safety is in progress.32 First year results for GALE were recently announced, which showed reduced GA lesion growth of 35% with monthly injections and 24% with every-other-month compared with the projected sham arm.33
Safety concerns arose in summer of 2023 when the American Society of Retina Specialists Research and Safety in Therapeutics Committee linked pegcetacoplan to six cases of occlusive retinal vasculitis.34 Retinal vasculitis was not observed in DERBY, OAKS or even the first year of GALE, though conversion to wet AMD remains the highest safety concern. Over three years, GALE found 19.5% cumulative risk of conversion to wet AMD in the monthly group compared to less than 9% in the every-other-month group.33
The second FDA approval arrived in August with Izervay (avacincaptad pegol).35 Izervay is a pegylated RNA aptamer targeting inhibition of C5, in turn decreasing the production of C5a and C5b.36 The FDA approval for monthly treatment was based on 18-month and 12-month data from the Phase III GATHER1 and GATHER2 clinical trials, respectively.
In total across the trials, 292 patients (vs. 332 with sham) were treated with an intravitreal injection of avacincaptad pegol (2mg) monthly.36 There was a 35% decrease in GA lesion growth in GATHER1 and 18% in GATHER2.36 Recently, Iveric Bio released 24-month data from GATHER2.37 The second year of the trial re-randomized patients to monthly or every-other-month treatment.37 At 24 months, the monthly treatment group had a 14% reduction in GA growth compared with a 19% reduction for the every-other-month group.37
There was one case of endophthalmitis and non-serious intraocular inflammation. There were no cases of retinal vasculitis or ischemic optic neuropathy. The rate of choroidal neovascularization was 12% in treated patients compared with 9% in the sham group.37 Of note, the GATHER trials evaluated only those with extrafoveal GA lesions. As such, treatment results cannot be directly compared with trials of pegcetacoplan, which additionally included fovea-involving GA lesions.
Both treatments attack central components of the complement system. Other therapeutic strategies, by comparison, have targeted complement factors, the regulators of the system. Both drugs in trial have demonstrated decreased GA lesion growth, although some have criticized these results as meager.27
Unfortunately for patients, none of these trials showed an improvement to visual function for the trade-off of frequent in-office treatments.27 Rather the goal with these treatments is to preserve vision for a longer time period, which can be a difficult concept for patients to grasp when they don’t recognize immediate gains as in exudative AMD treatments. On an encouraging note, both drugs have demonstrated that the treatment effect over placebo improved with treatment duration.
Additionally, a concern for both treatments is the increased risk of choroidal neovascularization. How that occurs is unclear. Is there too much shut-off of the complement system? Some have suggested that polyethylene glycol, present in both therapies, may be the culprit, as it has demonstrated in mouse models to induce CNV.38 Real-world data will be telling as both of these treatments have now entered retina specialist practice.
The long-awaited treatment for geography atrophy has finally arrived. For optometry, understanding the complement system in AMD development and complement inhibition with GA treatment helps to better serve our patients with GA.
Dr. Bedwell is a clinical associate professor at Indiana University School of Optometry. She is a fellow of the American Academy of Optometry and the Optometric Retina Society, as well as a member of the American Optometric Association. She also serves as editor of the Optometric Retina Society’s e-newsletter. She is a consultant for Iveric Bio.
1. Male D, Stokes PR, Male V. Immunology. 9th ed. Elsevier. 2021. 2. Mukai R, Okunuki Y, Husain D, et al. The complement system is critical in maintaining retinal integrity during aging. Front Aging Neurosci. 2018;10:15. 3. Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95-112. 4. Loyet KM, Deforge LE, Katschke KJ Jr, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(10):6628-37. 5. Khandhadia S, Cipriani V, Yates JR, et al. Age-related macular degeneration and the complement system. Immunobiology. 2012;217(2):127-46. 6. Harboe M, Ulvund G, Vien L, et al. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439-46. 7. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705-32. 8. Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-43. 9. Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151-71. 10. Seddon JM. Macular degeneration epidemiology: nature-nurture, lifestyle factors, genetic risk and gene-environment interactions - The Weisenfeld Award lecture. Invest Ophthalmol Vis Sci. 2017;58(14):6513-28. 11. Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45(11):1366-70. 12. Schramm EC, Clark SJ, Triebwasser MP, et al. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61(2):118-25. 13. Shughoury A, Sevgi DD, Ciulla TA. Molecular genetic mechanisms in age-related macular degeneration. Genes (Basel). 2022;13(7):1233. 14. Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433-9. 15. Sofat R, Casas JP, Webster AR, et al. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int J Epidemiol. 2012;41(1):250-62. 16. Lachmann PJ. The amplification loop of the complement pathways. Adv Immunol. 2009;104:115-49. 17. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-35. 18. Yates JR, Sepp T, Matharu BK, et al. Genetic Factors in AMD Study Group. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553-61. 19. Zhang J, Li S, Hu S, et al. Association between genetic variation of complement C3 and the susceptibility to advanced age-related macular degeneration: a meta-analysis. BMC Ophthalmol. 2018;18(1):274. 20. Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131(3):383-92. 21. Holliday EG, Smith AV, Cornes BK, et al. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS One. 2013;8(1):e53830. 22. Micklisch S, Lin Y, Jacob S, et al. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J Neuroinflammation. 2017;14(1):4. 23. Awh CC, Lane AM, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317-23. 24. Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology. 2015;122(1):162-9. 25. Vavvas DG, Small KW, Awh CC, et al. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc Natl Acad Sci USA. 2018;115(4):E696-E704. 26. Mukamal R. Genetics and age-related macular degeneration. American Academy of Opthalmology. www.aao.org/eye-health/diseases/age-related-macular-degeneration-amd-genetics. Accessed January 12, 2024. 27. Spaide RF, Vavvas DG. Complement inhibition for geographic atrophy: review of salient functional outcomes and perspective. Retina. 2023;43(7):1064-9. 28. Park B. Syfovre approved for geographic atrophy secondary to AMD. Medical Professionals Reference. February 21, 2023.Accessed January 12, 2024. 29. Syfovre (pegcetacoplan injection) [package insert]. Waltham, MA: Apellis Pharmaceuticals, Inc; 2023. 30. Steinle N, Boyer DS, Mones J, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the Phase III OAKS and DERBY studies. Invest Ophthalmol Vis Sci 2022;63(7):1500. 31. Wykoff, C. Treatment of geographic atrophy secondary to AMD With pegcetacoplan: two-year outcomes from the randomized Phase III OAKS and DERBY Trials. Presented at the American Academy of Ophthalmology Annual Meeting 2022. 32. An extension study to evaluate the long-term safety and efficacy of pegcetacoplan (APL-2) in subjects with geographic atrophy secondary to AMD (GALE). August 31, 2023. clinicaltrials.gov/ct2/show/NCT04770545. Accessed January 12, 2024. 33. Wykoff CC, Heier JS, Jones D, et al. Long-term efficacy and safety of pegcetacoplan from the GALE open-label extension of the Phase III OAKS and DERBY Trials. Presented at the American Academy of Ophthalmology Annual Meeting 2023. 34. Hutton, D. ASRS reports six cases of occlusive retinal vasculitis linked to pegcetacoplan injection. Ophthalmology Times. July 19, 2023. www.ophthalmologytimes.com/view/asrs-reports-six-cases-of-occlusive-retinal-vasculitis-linked-to-pegcetacoplan-injection. Accessed January 12, 2024. 35. Park B. Izervay approved for geographic atrophy secondary to AMD. Medical Professionals Reference. August 7, 2023.Accessed January 12, 2024. 36. Izervay (avacincaptad pegol intravitreal solution) [package insert]. Parsippany, NJ: IVERIC Bio, Inc; 2023. 37. Khanani AM, Patel SS, Staurenghi G, et al. GATHER2: two-year data. Presented at the American Academy of Ophthalmology Annual Meeting 2023. 38. Lyzogubov VV, Bora NS, Tytarenko RG, et al. Polyethylene glycol induced mouse model of retinal degeneration. Exp Eye Res. 2014;127:143-52. |
