Contact Lens-associated Red Eye: Causes and Corrections
These cases can be challenging, but they don't always have to be.
By Amanda Tompkins, OD
Release Date:
November 15, 2017
Expiration Date:
November 15, 2020
Goal Statement:
Contact lens-associated red eyes are among the most challenging presentations seen in everyday practice. However, with a detailed working knowledge of differentials, a thorough examination and swiftly established treatment plan, practitioners can confidently reach an accurate diagnosis and achieve the best possible outcome for their patients and ensure future success with contact lens wear.
Faculty/Editorial Board:
Amanda Tompkins, OD
Credit Statement:
This course is COPE approved for 1 hour of continuing education credit. Course ID is 55288-CL. Check with your state licensing board to see if this counts toward your CE requirements for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Authors: Dr. Tompkins has no financial relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Riviello and Michael Iannucci all have no relationships to disclose.
Red eyes are relatively common in practice but not always straightforward, and adding a contact lens to the ocular surface only complicates things. To avoid potentially vision-threatening scenarios, practitioners need to come up with a timely, appropriate diagnosis and treatment plan. This article discusses how to streamline these processes for contact lens-related red eyes to achieve the best possible outcome.
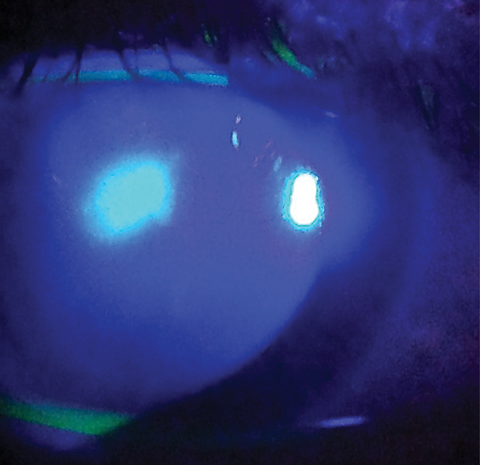 |
| In this MK infection, fluorescein is "spilling" into the surrounding stroma due to excavation of Bowman's layer, the relative size of the ulcer and the central location. Photo: Dan Fuller, OD |
Case History and Evaluation
Obtaining a thorough history for the patient right away should help steer the case in the right direction. The first step is to establish an appropriate list of differentials. Asking certain questions can help eliminate the most concerning etiologies and rule out several differentials. Some examples include:
• Can you pinpoint when this started?
• What is your wear schedule?
• What solution do you use?
• Do you have any medical conditions?
• Compared with yesterday, are your symptoms better, worse or about the same?
• How old is your lens storage case?
It is also crucial to know if a case of red eye started while the patient was wearing lenses, if there has been any recent change in environment such as swimming with lenses or being out of the country, if discomfort or pain increases upon lens removal and if there is pain, discomfort or itching in general. For the last point, patients will often say yes to all three. In those cases, practitioners can get them to prioritize their symptoms by asking: "If you could only tell me one thing that is bothering you, what would it be?"
Slit lamp observation. This is where working diagnoses are either confirmed or denied. When possible, these observations should be made with the patient's lens on. This will help determine if the red eye is from the lens itself or another etiology. From there, things to look for include lens deposits and defects, whether the lens is inside out and whether it fits properly.
Next is evaluation of the eyelashes, lid margins and tear film quality over the lens. This is a good time to look for any debris trapped under or on top of the lens and take note if there is a chance of poor hygiene with cosmetics or creams. A comprehensive eyelid examination should always include eversion of the lids to determine the presence of follicles or papillae over the tarsal plate.
After evaluation with the lens on, lens removal will allow for accurate analysis of the cornea and conjunctiva. From here, practitioners should look at the limbus for neovascularization. At this point, a corneal evaluation with and without staining is necessary. Before staining, it is important to complete a detailed corneal scan with both parallel pipette and optic sections with full illumination, when possible. Practitioners should evaluate each layer of the cornea to determine which are affected and if any edema or whitening of the cornea is present. Some conditions present with symptoms greater than signs and some may have very subtle signs. Another area in need of close inspection is the anterior chamber. The location, depth and size of any corneal deficiencies or defects will be helpful later, so it is prudent to take detailed notes.
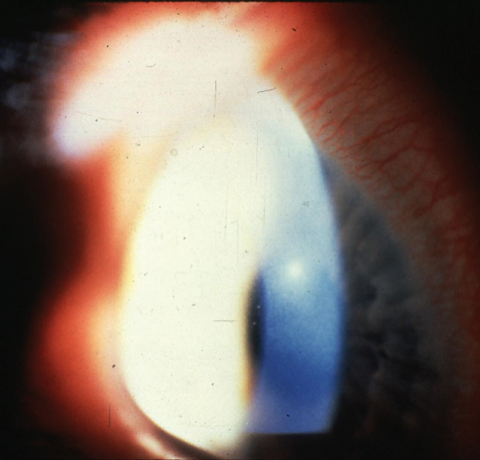
 |
| Here is an example of Acanthamoeba keratitis with deep stromal disease. |
Differential Diagnoses and Etiologies
Corneal infiltrates can be one of the most diagnostically challenging anterior segment conditions practitioners have to deal with. Their presentations and etiologies include both inflammatory and infective processes.
Inflammatory. Contact lens-induced acute red eye (CLARE) occurs in the presence of corneal hypoxia combined with noninvasive gram-negative bacteria that elicit an inflammatory reaction secondary to bacterial endotoxin.1 No actual corneal infection exists in this case. Symptoms include discomfort, contact lens intolerance and possibly mild pain. One key diagnostic feature is that lens removal relieves symptoms.
CLARE presentation can be unilateral or bilateral and consists of mild to moderate conjunctival hyperemia with associated corneal infiltrates located in the periphery to midperiphery. The infiltrates have no associated epithelial defect, distinguishing CLARE from both contact lens-induced peripheral ulcer (CLPU) and microbial keratitis (MK).2
Risk factors for CLARE include extended wear with poor replacement schedule, deficient lens and storage environment hygiene. Of note, H. influenza, a gram-negative bacteria, can colonize in the nasopharynx and lead to CLARE in patients who have recently been sick with the flu.3
In CLPU, inflammation occurs as the body responds to bacterial exotoxins from the colonization of gram-positive bacteria, namely, Staphylococcus species, on the surface of the contact lens.4
CLPU presentations are generally mild. In some cases, the condition may go undetected by the patient, and the clinician will observe the inactive infiltrate during routine evaluation. When symptomatic, patients may present with discomfort, foreign body sensation and tearing unilaterally.
Often, CLPU cases are associated with an epithelial defect, but Bowman's layer remains intact so there is minimal spillover of fluorescein into the surrounding stroma. Another key feature of CLPU is that the epithelial defect is usually considerably smaller that the stromal infiltrate and located in the mid-periphery or periphery. The biggest differential with CLPU is MK. CLPU is most similar to a very early stage of MK. Unlike MK, CLPU is typically not associated with photophobia, conjunctival hyperemia or anterior chamber reaction.5
Risk factors for CLPU include sleeping in lenses, prolonged replacement schedule and poor hygiene.
Contact lens-induced papillary conjunctivitis (CLPC) is included in discussions of giant papillary conjunctivitis (GPC), allergic conjunctivitis and vernal conjunctivitis.6 The pathophysiology of CLPC has been debated and conflicting theories still exist.7,8
 |
| This case of MK was caused by Pseudomonas aeruginosa. Note the large central ulcer and gross hyperemia. Photo: Elmer Tu, MD |
For this discussion, we will consider CLPC primarily to be an inflammatory condition initiated by protein deposits on the lens surface with an associated mechanical component.9 The deposition of protein on the lens surface depends on water content of the lens (higher water content means more deposition), as well as polymer content, structure and charge.1,10,11 Studies have documented that lenses with both high water content and ionic properties have the highest amount of protein deposits.12 The palpebral conjunctiva undergoes an inflammatory response mediated by a type I hypersensitivity reaction with immunoglobulin E components and a type IV hypersensitivity reaction in response to the presence of these proteins.13
Additionally, the movement of the tarsal plate over the edge of the lens contributes to repeated trauma of the tarsal conjunctiva. This results in a papillary reaction mediated by several inflammatory factors.
CLPC symptoms include lens intolerance and awareness, decreased wear time, blurred vision and an urge to excessively clean the lenses due to discharge.14 Signs include bilateral, large palpebral papillae on the upper palpebral conjunctiva, mucous discharge and excessive lens movement on inspection.7 Patients with CLPC may report symptoms are worse upon removal of the lens. The age of the contact lenses, frequency of replacement and length of extended wear period, as well as cleaning and case hygiene habits, contribute to increased risk of CLPC.
Contact lens-related hypersensitivity reactions related to lens care systems, drops being used or lens material (a silicone hydrogel combination with certain solutions) are another common source of contact lens-related red eyes.15 These patients often present with bilateral discomfort and hyperemia. Presentation is also characterized by diffuse superficial punctate keratitis.
Determining the source requires specific questioning regarding the pattern of their symptoms, recent changes in products they are using and an increase in artificial tear usage. Additionally, some clinicians suggest changing one variable at a time if the cause is unknown. Lens removal and copious amounts of preservative-free artificial tears will help alleviate symptoms and reduce superficial punctate keratitis (SPK) initially, but determining and eliminating the source is necessary for a long-term solution.
Infectious. MK is less common but more severe than sterile etiologies for contact lens-associated red eyes, so it must be considered as well. It is helpful to assume a case is microbial until proven otherwise. Contact lens-related infectious ulcers are typically caused by bacteria, fungi or protozoa.5
Bacterial MK is often caused by Pseudomonas aeruginosa.7,16 In early stages, the condition may mimic CLPU both in signs and symptoms, but quickly progresses. Patients with MK typically present with unilateral pain, photophobia and reduced vision, and a history of poor hygiene and contact lens over-wear.
 |
| A mid-peripheral corneal ulcer caused by Staphylococcus aureus. |
Signs include anterior chamber reaction and moderate to severe conjunctival hyperemia. Ulcers are generally located centrally and associated with an epithelial defect that is characteristically 1:1 with the stromal infiltrate, larger than 2.0mm and irregular in shape. Because of a break in Bowman's layer, instilled fluorescein usually spreads through the stroma beyond the stromal haze. There may also be satellite lesions surrounding the ulcer, which are absent in CLPU.5 Complicating differentiation from CLPU, both CLPU and MK ulcers can present in the midperiphery, and early MK ulcers are not always 1:1 with the stromal infiltrate.
The culprits in fungal infections typically include Aspergillus, Candida or Fusarium species.7,8 Classically, these infections are associated with an injury involving vegetable matter, but can also be associated with contact lens wear.
Patients often present with unilateral pain, photophobia and reduced vision. They may say their symptoms have been gradually increasing over the last several days to weeks. This is secondary to the slow rate of fungal growth, differing from the acute nature of bacterial infections.
The characteristic sign of a fungal infection is a central corneal infiltrate with "feathery" borders and associated satellite infiltrates.17 As with bacterial keratitis, an epithelial defect may be present over the stroma, but here, the epithelium may be elevated over the infiltrate, possibly intact.
On the protozoan front, Acanthamoeba must always be ruled out due to its refractory course and risk for permanent vision loss with these infections.7,8 Risk factors for this infection include poor contact lens hygiene from tap water use or exposure to hot tubs, swimming pools or other sources of contaminated water.7,18
Classically, these patients present with symptoms greater than signs. They usually show up at the office as a result of severe ocular pain and photophobia. Signs include conjunctival redness, dendritiform lesions resembling herpes simplex virus on the epithelium of the cornea in the early stage and a characteristic "ring" infiltrate in more advanced cases.5
Treatment and management
Once an accurate diagnosis is made, focus can shift to developing a treatment plan:
Inflammatory. With all inflammatory etiologies, removing the inflammatory trigger is the first step in treatment. This requires discontinuing contact lens wear until signs and symptoms are resolved. Often, removing the offending agent is sufficient for relief of symptoms, but expediting the process and returning patients to a comfortable state as quickly as possible is a good way to provide reassurance. The addition of an artificial tear will help aid in comfort and return the ocular surface to physiological equilibrium. For CLARE and CLPC, adding a topical steroid such as prednisolone acetate 1% can be helpful (in the absence of epithelial defects). While dosage varies with the severity of the condition, QID is often a good starting point.
Considering CLPU, it's best to assume these cases are MK until proven otherwise and treat with a fourth-generation topical fluoroquinolone. Generally, these cases do not need a loading dose, and TID dosing should suffice. Practitioners should see these patients the next day and on a 24-hour basis until there is resolution of the epithelial defect, at which point adding a steroid will reduce residual scarring. Alternatively, a topical combination drop would work as initial treatment.
For patients who are symptomatic for itching in cases of CLPC, a topical mast cell stabilizer (MCS) or MCS/antihistamine combination is necessary. If the patient's initial treatment includes a steroid, practitioners should avoid the addition of a MCS/antihistamine combination at first because of the anti-inflammatory effects of the steroid. In these cases, a combination drop can be added later and is often beneficial when used as prophylactic treatment for recurrent cases.
In all inflammatory cases of red eye, it is important to continuously follow up with patients and make sure they are not wearing their lenses until symptoms have subsided. At that point, they can resume lens wear on a titrated schedule. In the case of CLPC, papillae may take months to resolve. Here, a daily wear option may prevent recurrence, long-term. In cases where daily disposable lenses are not an option, a hydrogen peroxide-based care system with emphasis on zero tolerance for extended wear or extended replacement schedule will greatly reduce the risk of recurrence.
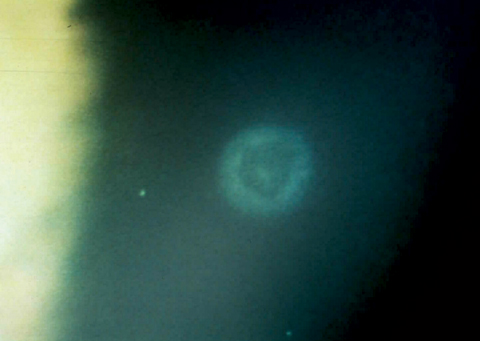 |
| A healed CLPU several months after initial onset. |
Infectious. For MK, broad-spectrum, fourth-generation fluoroquinolones such as gatifloxacin, besifloxacin and moxifloxacin are the best options for treatment.19,20 These offer the most broad-spectrum antibiotic properties and are valuable in cases when the organism is unknown. Practitioners should consider a loading dose for all suspected bacterial keratitis cases.21 Loading dosages vary but can be anywhere from every 15 minutes for the first hour up to every five minutes for the first 30 minutes depending on severity of the ulcer. To determine the organism, culturing and fortification of antibiotics may be required. Additionally, referral to a corneal specialist may be required in severe, visually threatening cases.22
Fugal keratitis is notoriously difficult to treat, and the fact that only one commercially available topical antifungal agent, natamycin, exists complicates the treatment plan. Others are available, but in recent studies natamycin provided significant improvement over fortified voriconazole.11 It is prudent to begin treatment once a fungal etiology is suspected, but referring to a corneal specialist for comanagement can also be a helpful option in these cases.23
Acanthamoeba is a protozoa, so it is not successfully treated with antibiotics. Because of this, and given the organism's aggressive nature, antiseptics are necessary.24 The most commonly used antiseptics are polyhexamethylene biguanide and chlorhexidine, both of which have proven effective in clinical trials.18,25
After initiating treatment for any of these conditions, practitioners should follow up with patients the next day and then every one to two days until there is consistent improvement. A cautious taper of medication is recommended to best prevent recurrent infection. Fortified topical antibiotics, such as tobramycin and vancomycin, and fortified antifungals, such as voriconazole, may be necessary in the case of large or central ulcers or those not responding to already aggressive use of antimicrobial medication. Again, comanagement is often helpful in these cases.
When to Culture
Typically, when dealing with an ulcer that is small or in the periphery empirical treatment with broad-spectrum antibiotics is sufficient to yield stabilization and resolution of the infection. However, for lesions that either don't respond to empirical treatment, are greater than 2mm in size or are in the central cornea, culturing is indicated. Additionally, infiltrates suspected to be secondary to an unusual organism, such as nosocomial infections or those originating from vegetative trauma, should be cultured so the appropriate treatment can be initiated as quickly as possible.
Contact lens-associated red eyes are among the most challenging presentations seen in everyday practice, and the wide spectrum of etiologies only complicates diagnosis and treatment. However, with a strong working knowledge of the differentials, a detailed examination and swiftly initiated treatment, practitioners can confidently reach an accurate diagnosis and achieve the best possible outcome, securing a successful future of contact lens wear for patients.
Dr. Tompkins is an instructor at The Eye Center at Southern College of Optometry. Her professional interests are in primary care, glaucoma, diabetes, clinical and didactic education as well as local and international medical mission work.
1. Holden BA, La Hood D, Grant T, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22(1):47-52. |
