Can You Spot These Retinal Vascular Abnormalities?
Abnormal changes in the retinal vasculature are closely linked with underlying systemic conditions—impacting both the physical and ocular health of the patient.
By Nick Fogt, OD, PhD, and Theresa Watt, BS
Release Date: March 15, 2019
Expiration Date: March 15, 2022
Estimated Time to Complete Activity: 2 hours
 |
Jointly provided by Postgraduate Institute for Medicine (PIM) and RGVCE
Educational Objectives: After completing this activity, the participant should be better able to:
- Evaluate ocular signs, symptoms and pathophysiology of retinal vascular abnormalities.
- Identify changes in retinal vascular architecture, such as increased retinal vein caliber, retinal vascular tortuosity, increased prominence of the retinal arterial reflex, venous nicking, “copper” or “silver wire” appearance, as well as the detection of cholesterol, calcium or thrombotic emboli.
- Provide management, additional testing or referral to prevent vision loss and possible systemic impairment.
- Offer patient education regarding potential retinal, systemic, neurological and cerebral vascular morbidities and risks.
Target Audience: This activity is intended for optometrists engaged in the care of patients with retinal vascular abnormalities.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and RGVCE. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Nick Fogt, OD, PhD, associate professor, Illinois College of Optometry, and Theresa Watt, BS.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 61580-PS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Fogt and Ms. Watt have nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The RGVCE planners, managers and editorial staff have nothing to disclose.
The retinal vasculature provides clinicians the unique opportunity to directly and noninvasively evaluate changes in the eye that reflect the systemic health of the patient. Classic examination techniques such as direct and indirect ophthalmoscopy, in addition to developed and newly developing retinal imaging devices, allow optometrists to detect subtle changes in retinal vasculature.
Here’s a summary of the relationships between changes in systemic health and the retinal vasculature.
Basic Retinal Vascular Anatomy
To properly navigate abnormal retinal vasculature, it’s helpful to first review the normal retinal vascular anatomy:
• Retinal blood supply. The retinal blood supply is provided by the central retinal artery (CRA) and the choroidal blood vessels.1 These two systems originate from the ophthalmic artery, which branches from the internal carotid artery. The inner retinal layers are nourished by the CRA, which courses along the boundary of the optic nerve sheath and enters via the optic nerve head. The other sources of blood supply are the choroidal vessels, which originate from the posterior ciliary artery branches of the ophthalmic artery to supply the outer retinal layers.
As the CRA projects though the optic nerve, the artery divides to form superior and inferior branches. Subsequently, these branches divide into the superior nasal and superior temporal arteries and the inferior nasal and inferior temporal arteries, serving the four quadrants of the retina.1,2 The extensive arterial network progressively branches until arriving at the ora serrata. The retinal venous circulation also has branches in these four quadrants, and these venous branches ultimately drain into the central retinal vein, which leaves the eye in the optic nerve.
In about 70% of arteriovenous (AV) crossings, the arteriole is “on top” (that is, on the vitreal side) of the venule.3 The arteriole and venule share a common adventitial sheath at these crossings. Branches from the central retinal vessels form capillary layers in the nerve fiber layer, ganglion cell layer and the inner nuclear layer. The outer plexiform and photoreceptor layers contain no blood supply from the CRA branches and are therefore nourished by the choroid.
About 15% to 20% of individuals possess a cilioretinal artery derived from the short posterior ciliary arteries, which courses toward the fovea and augments the retinal vasculature between the optic nerve head and the macula.1
• Choroidal blood supply. The choroidal vessels are derived from the ophthalmic arteries.4 The major branch of the internal carotid divides into the left and right ophthalmic arteries. The ophthalmic arteries extensively branch into the posterior ciliary arteries as they advance toward the globe.
Further branching gives rise to the short posterior ciliary arteries supplying the posterior choroid and the long posterior ciliary arteries, which nourish the anterior portion of the choroid.
The short posterior ciliary arteries also branch into the choriocapillaris. From the choroid, venous circulation exits the eye through the vortex veins in each quadrant of the retina. The vortex veins drain into the superior and inferior ophthalmic veins, which leave the eye and enter the cavernous sinus.
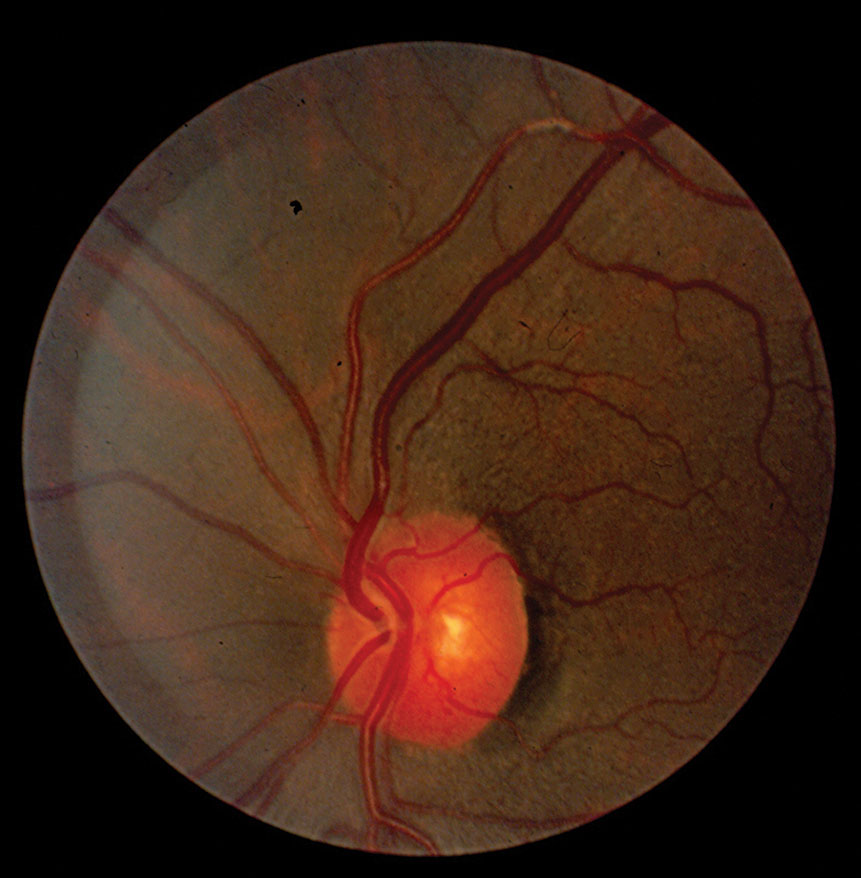 |
| Fig. 1. Increased reflectivity from the arteriole vessel wall and vascular tortuosity. |
General Vascular Pathophysiology
Abnormal changes in the retinal vasculature are closely tied to underlying systemic conditions.5-15 Some important risk factors for alterations in the retinal vasculature include diabetes, hypertension, hyperlipidemia, smoking, obesity and sedentary lifestyle. The likelihood of encountering these vascular changes increases with age.
Systemic vascular abnormalities bring about vascular remodeling.16,17 In this process, changes in hemodynamic conditions result in alterations of the vasculature. These alterations may include changes in vessel diameter, changes in the diameter of the vessel lumen, thickening of the vessel wall (which can result in a decrease in vessel lumen diameter) or atherosclerosis (with a subsequent reduction in luminal diameter).
Disruption of the blood-retinal barrier may ultimately occur, resulting in vascular leakage including hemorrhages, hard exudates and edema.
Atherosclerosis develops from a buildup of plaques on the lumen of vessel walls. This occurs in response to uncontrolled diabetes, hypertension, hypercholesterolemia and many other systemic disorders.
Traditionally, the development of atherosclerotic disease was described in terms of endothelial cell damage, which led to smooth muscle proliferation and then deposition of low-density lipoproteins (LDL) to the lumen walls.16 More recently, inflammation is recognized as a major contributor to atherosclerosis.18 As deposition of material increases, hard plaques increase in thickness and present an emergent risk of thrombus formation. Rupture of these plaques or thrombi can lead to stroke and increased mortality.
While atherosclerosis is described as a risk factor in some of the discussion below, remember that the atherosclerotic process is often accompanied by an underlying systemic disease.
 |
| Fig. 2. Proliferative diabetic retinopathy with intraretinal hemorrhages, a preretinal hemorrhage and hard exudates. Click to enlarge. |
Specific Retinal Vascular Abnormalities
Highlighted below are characteristic retinal changes that are important to recognize:
• Tortuosity. This vascular anomaly can be seen in both retinal arteries and veins and their successive branches (Figure 1). Systemic vascular diseases can alter the stability of blood vessel walls, which then results in changes in the course or shape of the vessels.11 In terms of systemic vascular diseases, retinal vascular tortuosity is primarily associated with hypertension and atherosclerosis.
• Changes in vessel caliber. Dilated retinal venules (perhaps associated with increased retinal venous pressure) and narrowed retinal arterioles (perhaps due to vessel attenuation associated with high lumen pressure) may occur and are potentially associated with a number of ocular and systemic disorders as described below.10
• Retinal hemorrhages. Breakdown of the blood-retinal barrier can lead to blood leakage (Figure 2). These hemorrhages can present in a multitude of appearances and may occur anywhere within the retina.8,19 Dot-and-blot hemorrhages are small, often circular in appearance and can be found in the inner nuclear and outer plexiform layers of the retina. Flame-shaped hemorrhages follow the course of the nerve fiber layer and may have a splinter-like appearance. Hemorrhages may also appear in the preretinal (between the internal limiting membrane and the nerve fiber layer) and subhyaloid (between the internal limiting membrane and the posterior boundary of the vitreous) spaces. These latter hemorrhages may present with a boat-shaped appearance. Vitreous hemorrhages may also occur.
• Widening of the arterial light reflex. This emanates from the junction between the blood column and the retinal arterial wall. A wider reflex manifests as a result of wall thickening/hardening due to persistent sclerosis, or perhaps as a result of reduction in the velocity of blood flow through the vessels (Figure 1).7 Chronic hypertension is one of the main culprits for this presentation, which researchers describe as a “copper” or “silver wire” appearance. When the reflex has a red-orange hue, this is referred to as copper wiring. Silver wire vessels give a white reflection from advanced sclerotic sheathing surrounding the arterial wall. In one study, severe enhancement of the arterial light reflex was associated with high blood pressure.7 However, the same study found no relationship between an enhanced arterial light reflex and mortality rate.7
• Emboli. These may travel to the retinal vasculature (Figure 3). Emboli are potentially composed of cholesterol, calcium and platelet-fibrin. Cholesterol emboli often appear as highly reflective, small, bright yellow or white spots within retinal vessels and are associated with atherosclerotic disease that includes the carotids.20 Calcific emboli appear as larger white deposits and typically are released from calcified heart valves.21 Platelet-fibrin emboli are less reflective, dull white-grey deposits.22 Talc retinopathy is another embolitic finding, predominantly caused by injected illicit drug use that had talc as a filler agent.23
• Arteriovenous crossing changes. Due to the typical anatomical position of arteries overlaying veins in the retina, changes in blood vessel wall cellular composition can create a “banking” or “nicking” appearance at arteriovenous crossings. This is due to arterial compressive impingement on the surface of the veins, as the arteries and veins share an adventitial sheath at these crossings.3
• Neovascularization. Also referred to as angiogenesis, neovascularization is the anatomical growth of new blood vessels due to hypoxic conditions. Oxygen-deprived tissue results in increased levels of various platelet and vascular endothelial growth factors (VEGF).24 These initiate the development of abnormal and fragile blood vessels in this area.
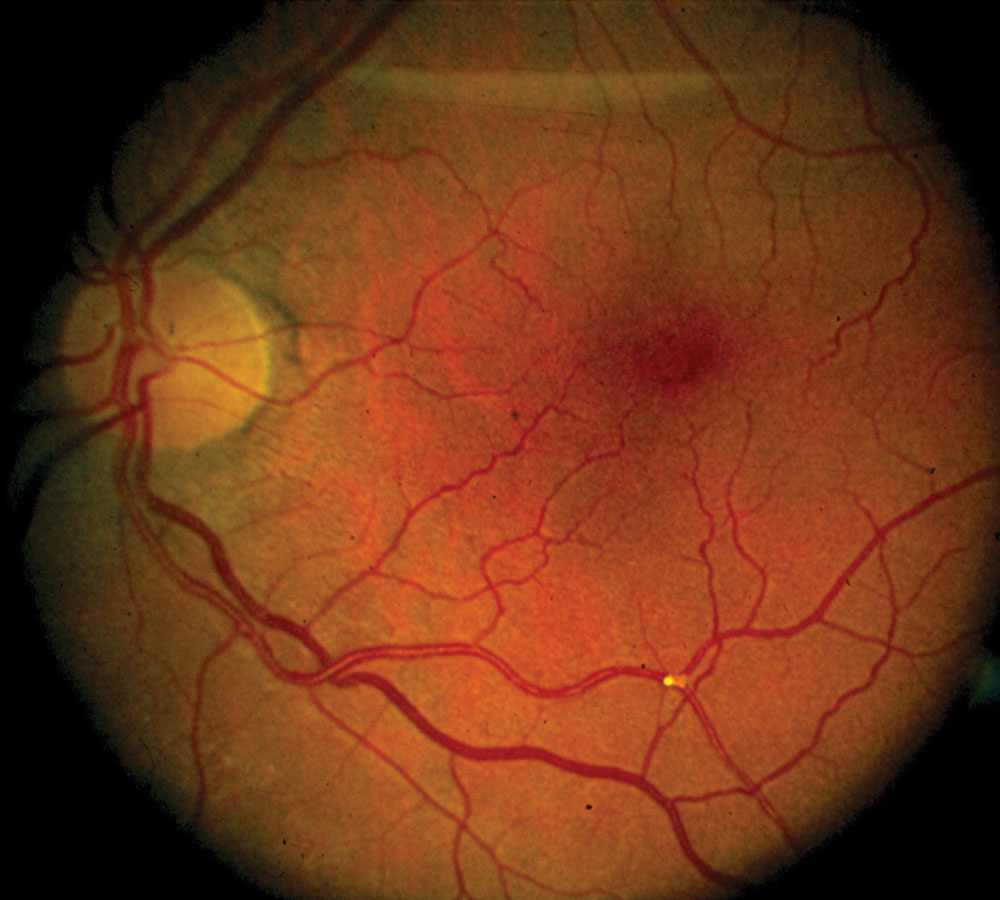 |
| Fig. 3. Retinal embolus with vascular tortuosity. Click to enlarge. |
Pathophysiology of Diabetes and Hypertension
Because diabetes and hypertension are commonly encountered in clinical practice, some details related to the pathophysiology of these disorders are discussed here:
• Diabetes. Increased blood glucose levels contribute to lipid deposition in vessels walls, ultimately resulting in atherosclerotic vascular changes and decreased perfusion to the retina. Primary changes in retinal vasculature due to diabetes include reduction in pericytes in the retinal capillaries and vascular cell apoptosis leading to impaired blood supply to the retina. Natural mechanisms then upregulate angiogenic factors such as VEGF to generate new vascular supplies to the retina. However, these neovascular vessels are prone to leakage.25 Vision loss may ensue from bleeding, edema or epiretinal membrane (ERM). Major risk factors for diabetic retinopathy are duration of diabetic diagnosis and blood glucose control.26 Diabetic patients with hypertension also show reduced diabetic retinopathy progression when blood pressure is tightly controlled.2
• Hypertension. Chronically elevated blood pressure can also lead to atherosclerotic changes in the retinal vasculature. Normal blood pressure is defined as a systolic pressure less than 120mm Hg and a diastolic pressure less than 80mm Hg.27,28 Hypertension is associated with endothelial cell damage and deterioration of arteriole smooth muscle.29,30 Destruction of the vessels walls is followed by plasma leakage and vascular necrosis.31 The resultant narrowing of the vascular lumen leads to systemic ischemic events, and in the eye promotes vascular infarcts (cotton-wool spots) and vascular leakage (hemorrhage, hard exudate, edema). The major risk factors for hypertensive retinopathy are the duration of hypertension and the level of blood pressure elevation.32
Retinal Vascular Changes and Systemic Health
Several conditions other than diabetes and hypertension can also affect the retina:
• Metabolic syndrome. This involves a group of five risk factors that include a large waistline (abdominal obesity), a high triglyceride level, a low high-density lipoprotein (HDL) level, high blood pressure and high fasting blood glucose. A person with three or more of these risk factors is diagnosed with metabolic syndrome. The risk factors associated with metabolic syndrome promote the development of atherosclerotic disease (at least partially by activating immune responses); as the number of these risk factors increases, the risk for coronary heart disease, stroke and diabetes also increase.33
Not surprisingly, retinal vascular changes that are potentially associated with risk factors for metabolic syndrome have also been associated with risk for coronary heart disease and stroke. This is not to say that risk factors for metabolic syndrome are the only factors associated with these vascular changes, but rather that clinicians should be aware that the combination of risk factors associated with metabolic syndrome may result in additive systemic and ocular effects.
• Narrowed retinal arterioles and larger retinal venules. Narrowed arterioles and larger venular diameters (which are linked with higher levels of systemic inflammation and vascular endothelial cell dysfunction) have been associated with the risk for coronary heart disease in a number of studies.10
For instance, the Atherosclerosis Risk in Communities (ARIC) Study demonstrated that lower arteriole-to-venule (AVR) ratios were predictive of coronary heart disease in women but not in men.34 A recent report from the ARIC group concluded that narrower retinal arterioles and wider retinal venules are associated with greater risk of mortality and stroke in men and women and a greater risk for coronary heart disease in women.35 An analysis of combined data from the Blue Mountain Eye Study and the Beaver Dam Eye Study concluded that both narrower arterioles and wider venules are predictive of higher risk for mortality associated with coronary heart disease and stroke in people between 43 to 69 years old.36 A meta-analysis also demonstrated an association between narrowed retinal arterioles and dilated retinal venules and coronary heart disease in women but not in men.37
Some researchers have suggested that quantifying the AVR can be useful in classifying patients for their future risk of cardiovascular events, although the impact in clinical practice of assessing the AVR ratio for stratifying patients for cardiovascular risk may be relatively modest.10,35,38 In any event, the AVR ratio can certainly be useful for eyecare practitioners to assess the presence and perhaps the extent of systemic cardiovascular disease.
• Microvascular findings. Other vascular changes associated with systemic disorders may also be predictive of coronary heart disease. One such group of findings is arteriovenous crossing changes (e.g., nicking and banking) and arteriolar narrowing. A second group is those associated with retinopathy, including microaneurysms, vascular leakage (hemorrhage or lipid exudate) or vascular blockage (cotton-wool spot) and neovascularization. The results of a study examining cases from the Beaver Dam Eye Study (ages 43 to 84) showed that retinopathy (including microaneurysms, intraretinal hemorrhages, hard exudates, cotton-wool spots, venous beading, intraretinal microvascular abnormalities, neovascularization, preretinal hemorrhages and vitreous hemorrhages) was related to cardiovascular mortality, while only middle-aged persons showed associations with other retinal findings (arteriovenous crossing changes and arteriolar narrowing) and cardiovascular risk.6
• Retinal arteriolar emboli. These are associated with the risk of future cardiovascular events, particularly stroke.39 These emboli may be derived from cardiac plaques or carotid artery plaques. Research shows hypertension, diabetes and cigarette smoking may all be associated with incident retinal emboli.
Because retinal emboli may be associated with systemic and ocular risks (i.e., retinal artery occlusion), patients with asymptomatic retinal emboli should be referred to a physician for a cardiovascular workup shortly after the ocular examination.
 |
| Fig. 4. Central retinal vein occlusion associated with systemic hypertension. Click to enlarge. |
Cardiovascular and Cerebrovascular Events
Specific retinal vascular disorders can also be related to cardiovascular or cerebrovascular complications:
• Hypertension and hypertensive retinopathy. We can use a three-grade classification scheme as proposed by researchers to categorize hypertensive retinopathy.8,40 The first grade is mild hypertensive retinopathy, which is characterized by changes in the arteriolar walls including widening of the arterial light reflex, arteriolar narrowing and arteriovenous crossing changes. The second grade, moderate hypertensive retinopathy, includes microaneurysms, vascular leakage (hemorrhages and hard exudates) and vascular occlusion (cotton-wool spots). The third grade, severe hypertensive retinopathy, includes any, or all, changes in moderate hypertensive retinopathy in addition to optic disc edema.
The choroid may also be affected by hypertension.
Moderate hypertensive retinopathy and to a lesser degree mild hypertensive retinopathy are associated with an increased risk for stroke. Hypertensive retinopathy may even be related to cardiac disease. Patients with findings of hypertensive retinopathy should have their blood pressure checked in the office and should be referred for a cardiovascular workup and treatment for their hypertension.
Hypertension can also predispose patients to retinal venous occlusions and retinal artery occlusions. Retinal vascular occlusions are the second most common sight-threatening retinal vascular disorder (diabetic retinopathy being the most common).15 While hypertension and hyperlipidemia are common systemic associations with vascular occlusion, many other potential systemic associations, including diabetes, atherosclerotic disease and hypercoagulation disorders exist.9,14 Certain classes of medications can also predispose patients to retinal venous occlusions.14
In retinal vein occlusions, the veins in the pre- and post-venous occlusion phases are often dilated and tortuous (Figure 4).8,41 Retinal hemorrhages are characteristic of retinal vein occlusions, and cotton-wool spots may appear. Post-venous occlusion changes in the retinal vasculature may include vascular sheathing and retinal or optic nerve head collateral vessels.
Management of retinal venous occlusions involves addressing the likely systemic factors underlying the occlusion and consulting with an ophthalmologist regarding the potential for macular edema, retinal neovascularization or, in some cases, iris neovascularization.
Retinal artery occlusions often result from emboli originating from the carotid artery or the heart. These occlusions are associated with a number of systemic risk factors in addition to carotid artery disease, such as hypertension, atherosclerosis, heart abnormalities and many hematologic and inflammatory issues.8 It is important to determine whether an arteritic inflammatory disorder is associated with the vascular occlusion.
Central retinal artery occlusion is a true ocular emergency, requiring immediate care in an attempt to restore circulation to the eye, although the efficacy of treatments (e.g., paracentesis, intraocular pressure-lowering drugs) is unclear.42 Branch retinal artery occlusion may be treated in a similar manner as central retinal artery occlusion, but carries a better visual prognosis.43,44
Recently, researchers have suggested that transient monocular vision loss attributable to vascular causes, branch retinal artery occlusion, and central retinal artery occlusion be grouped together under the term “acute retinal arterial ischemia.”45 In addition, acute retinal arterial ischemia has now been characterized as equivalent to stroke in the brain. Researchers recommend that all of these entities be managed in the same way. Specifically, once any of these disorders is diagnosed, the patient should be referred to an emergency department associated with a stroke center. This is because patients with these disorders are at significant risk for stroke and cardiac events, and the risk of these things occurring rises with delays in treating those issues (e.g., carotid stenosis, cardiac abnormalities) that lead to such events.45 In fact, the incidence of stroke is highest in the first week after a central retinal artery occlusion and remains high over the first 30 days after the occlusion.46
• Diabetes and diabetic retinopathy. In patients with both Type 1 and Type 2 diabetes, the presence of diabetic retinopathy is often associated with both neuropathy and kidney disease (albuminuria).47 The extent of diabetic retinopathy can therefore be taken as an indicator of the extent of systemic microvascular disease. Further, a literature review suggested that higher rates of all-cause mortality were found in Type 1 and Type 2 diabetic patients with diabetic retinopathy compared with diabetic patients without diabetic retinopathy.48 The relative risk was higher in patients with proliferative diabetic retinopathy compared with patients with nonproliferative diabetic retinopathy. Diabetic retinopathy was also associated with an increased risk of stroke and heart failure.
• Ocular ischemic syndrome. This occurs due to stenosis of the carotid artery (or rarely the ophthalmic artery) and subsequent reduction (hypoperfusion) of blood flow to the eye.12
Changes in the anterior and posterior segments may occur in ocular ischemic syndrome. In terms of vascular changes in ocular ischemic syndrome, laterality is a significant feature to consider. Ocular ischemic syndrome tends to be unilateral, while diabetic retinopathy tends to be bilateral. In ocular ischemic syndrome, the retinal veins may be dilated and non-tortuous, while the arteries may be narrowed. Retinal hemorrhages, particularly in the mid-periphery, may exist, along with microaneurysms in the mid-periphery and in the posterior pole.12,49 Macular capillary telangiectasias may also appear. Neovascularization of the retina is possible, and iris neovascularization is common.49
Ocular ischemic syndrome is caused primarily by atherosclerotic disease. The most common underlying systemic causes of atherosclerotic disease that lead to ocular ischemic syndrome are hypertension and diabetes. There is a high, five-year mortality rate associated with ocular ischemic syndrome, primarily related to cardiovascular disease and stroke.12 Thus, patients who manifest ocular ischemic syndrome should be referred for a cardiac and carotid evaluation.
Abnormal vascular findings in the retina are often harbingers of systemic disease that could lead to vascular events such as stroke. All of the retinal findings discussed here should prompt clinicians to refer patients for a systemic cardiovascular workup and discuss modifiable risk factors with the patient, such as smoking cessation and reduction in body mass index, perhaps through a combination of diet and exercise.
Dr. Fogt is a professor at the Ohio State University College of Optometry, where he teaches courses in posterior segment disease and systemic disease.
Ms. Watt graduated from the University of North Carolina at Chapel Hill and is currently a student at the Ohio State University College of Optometry (class of 2020).
| 1. Snell RS, Lemp MA. Clinical Anatomy of the Eye. Cambridge, MA: Blackwell Scientific Publications; 1989:171-175, 255. 2. Alexander L. Primary Care of the Posterior Segment. 2nd ed. Norwalk, CT: Appleton & Lange; 1994:219. 3. Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol. 1990 Mar 15;109(3):298-302. 4. Kiel JW. The Ocular Circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2010: ch 2 (Anatomy). 5. Yu T, Mitchell P, Berry G, et al. Retinopathy in older persons with diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116(1):83-9. 6. Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003 May;110(5):933-940. 7. Kaushik S, Tan AG, Mitchell P, Wang JJ. Prevalence and associations of enhanced retinal arteriolar light reflex: a new look at an old sign. Ophthalmology. 2007 Jan;114(1):113-120. 8. Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369(9559):425-35. 9. Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol. 2010 Jul 30;4:809-16. 10. Liew G, Wang JJ. Retinal vascular signs: A window to the heart? Rev Esp Cardiol. 2011;64(6):515-21. 11. Han HC. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res. 2012;49(3):185-97. 12. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome – a systematic review. Med Sci Monit. 2012;18(8):RA138-144. 13. Varner P. The clinical import of a retinal cholesterol embolus: it is a question of symptoms. Clinical Optometry. 2013:5:13-17. 14. Citirik M, Haznedaroglu IC. Clinical risk factors underlying the occurrence of retinal vein occlusion. Int J Ophthal Res. 2016:2(1):91-95. 15. Uchida A, Ehlers JP. Management trends in retinal vascular occlusive diseases. Retina Today. 2018 Apr;13(3):52-58. 16. Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994 May 19;330(20):1431-8. 17. Renna NF. de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353. 18. Lim S, Park S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014 Jan;47(1):1-7. 19. Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic retinopathy. Prim Care. 2015 Sep;42(3):451-64. 20. Rousseau A, de Monchy I, Barreau E, et al. Retinal emboli in cholesterol crystal embolism. Case Rep Ophthalmol Med. 2013;2013:421352. 21. Ramakrishna G, Malouf JF, Younge BR, et al. Calcific retinal embolism as an indicator of severe unrecognized cardiovascular disease. Heart. 2005 Sep;91(9):1154-1157. 22. Cho KH, Ahn SJ, Cho JH, et al. The characteristics of retinal emboli and its association with vascular reperfusion in retinal artery occlusion. Invest Ophthalmol Vis Sci. 2016 Sep 1;57(11):4589-98. 23. Zoumalan CI, Marmor MF. Revisiting talc retinopathy. Arch Ophthalmol. 2007 Jul;125(7):988. 24. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843-5. 25. Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013 Jan 8;17(1):20-33. 26. Fong DS, Aiello L, Gardner TW, et al; American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004 Jan;27(Suppl 1):S84-S87. 27. Schwartz CL, McManus RJ. What is the evidence base for diagnosing hypertension and for subsequent blood pressure treatment targets in the prevention of cardiovascular disease? BMC Med. 2015 Oct 12;13:256. 28. New ACC/AHA high blood pressure guidelines lower definition of hypertension. 2017 Nov 13. American College of Cardiology. www.acc.org/latest-in-cardiology/articles/2017/11/08/11/47/mon-5pm-bp-guideline-aha-2017. Accessed February 25, 2019. 29. Dharmashankar K, Widlanskyk ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010 Dec;12(6):448-55. 30. Touyz RM, Alves-Lopes R, Rios FJ, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018 Mar 15;114(4):529-39. 31. Garner A, Ashton N. Pathogenesis of hypertensive retinopathy: a review. J R Soc Med. 1979 May;72(5):362-5. 32. Erden S, Bicakci E. Hypertensive retinopathy: incidence, risk factors, and comorbidities. Clin Exp Hypertens. 2012;34(6):397-401. 33. Metabolic Syndrome. National Heart, Lung, and Blood Institute. www.nhlbi.nih.gov/health-topics/metabolic-syndrome. Accessed February 25, 2019. 34. Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002 Mar 6;287(9):1153-9. 35. Seidelmann SB, Claggett B, Bravo PE, et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: The Atherosclerosis Risk in Communities Study. Circulation. 2016 Nov 1;134(18):1328-1338. 36. Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007 Aug;28(16):1984-92. 37. McGeechan K, Liew G, Macaskill P, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009 Sep 15;151(6):404-13. 38. McGeechan K, Liew G, Macaskill P, et al. Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2008 Jul 1;102(1):58-63. 39. Klein R, Klein BE, Moss SE, Meuer SM. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol. 2003 Oct;121(10):1446-51. 40. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004 Nov 25;351(22):2310-7. 41. Lee DH, Lee SJ, Yoon lN. Clinical progress in impending central retinal vein occlusion. Korean J Ophthalmol. 2010 Apr;24(2):83-8. 42. Chronopoulos A, Schutz JS. Central retinal artery occlusion – a new, provisional treatment approach. Surv Ophthalmol. 2019 Jan 29. pii: S0039-6257(18):30215-7. 43. Muramatsu D, Minezaki T, Tsubota K, et al. Retrospective study of threshold time for the conventional treatment of branch retinal artery occlusion. Clin Ophthalmol. 2014 Sep 22;8:1877-81. 44. Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol. 2004 Sep-Oct;48(5):490-2. 45. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia. Follow the guidelines! Ophthalmology. 2018 Oct;125(10):1597-1607. 46. Park SJ, Choi NK, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015 Nov:122(11):2336-2343. 47. Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care. 1996 Nov;19(11):1243-8. 48. Zhu XR, Zhang YP, Bai L, et al. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure. Medicine (Baltimore). 2017 Jan;96(3):e5894. 49. Malhotra R, Gregory-Evans K. Management of ocular ischaemic syndrome. Br J Ophthalmol. 2000 Dec;84(12):1428-31. |
