 |
Addressing Pterygium in Optometric Practice
Successful management of this condition requires a comprehensive understanding of its pathophysiology, clinical features and diagnostic criteria.
By Elizabeth Chetty, DPhil, and Annelize van Zyl, MPhiL
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: November 15, 2023
Expiration Date: November 15, 2026
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists concerned with skin abnormalities in the ocular area.
Educational Objectives: After completing this activity, participants should be better able to:
Recognize the multifactorial pathophysiology of pterygium.
Identify the clinical factors associated with this condition.
Recognize the risk factors of pterygium and educate patients accordingly.
Effectively manage pterygium patients alongside a multidisciplinary team.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Chetty and Ms. van Zyl have nothing to disclose. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #127176 and course ID 88074-TD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
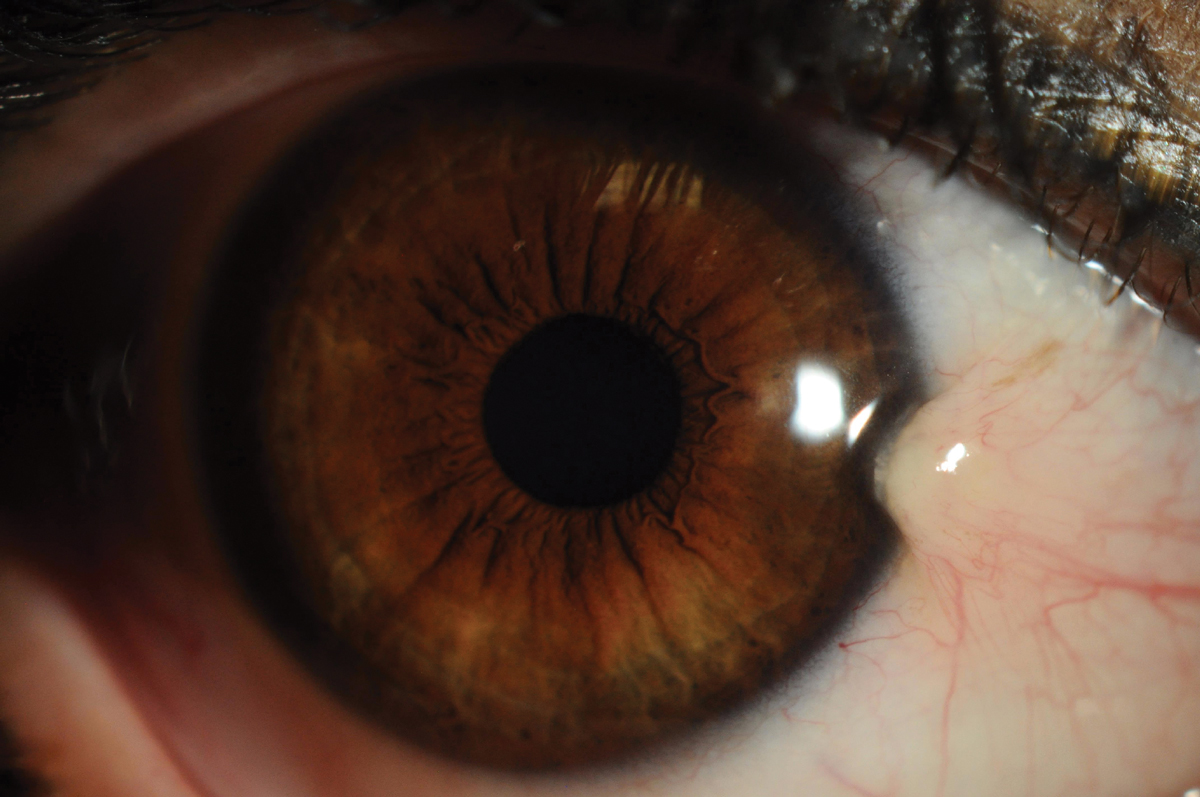 |
Fig. 1. Pterygium is a wing-like or triangular raised centripetal lesion that grows from the conjunctiva onto the cornea. Click image to enlarge. |
Pterygium, or surfer’s eye, is a common fibrovascular degenerative conjunctival condition associated with chronic exposure to ultraviolet (UV) radiation. The term pterygium is derived from the Greek word “pterygion,” meaning “wing,” which reflects the condition’s characteristic appearance: a wing-like or triangular raised centripetal lesion that grows from the conjunctiva onto the cornea (Figure 1). It is usually bilateral and found on the nasal aspect of the eye but can also be present on the temporal side as well.
The prevalence of pterygium globally averages 12%; however, the numbers vary vastly from 1.3% to 53%.1-3 Various risk factors such as demography and environment play a role in the discrepancy in reported prevalence, with higher numbers reported for older individuals living in sunny, dusty, equatorial regions or those with outdoor occupations.1
To fully understand our role as eyecare practitioners in managing this condition, it is necessary to understand its multifactorial pathophysiology, clinical features, diagnostic criteria and current management strategies.
Understanding Pathophysiology
While the pathophysiology is complex and poorly understood, there has been a long-standing association between prolonged UV radiation (sunlight) exposure and pterygium formation.4 Ultraviolet radiation is pivotal in initiating and promoting pterygium formation through several mechanisms, including inflammation, fibroblast activation, extracellular matrix remodeling, angiogenesis and tissue invasion. Genetic factors and viral infection have also been associated with the pathophysiology.5,6
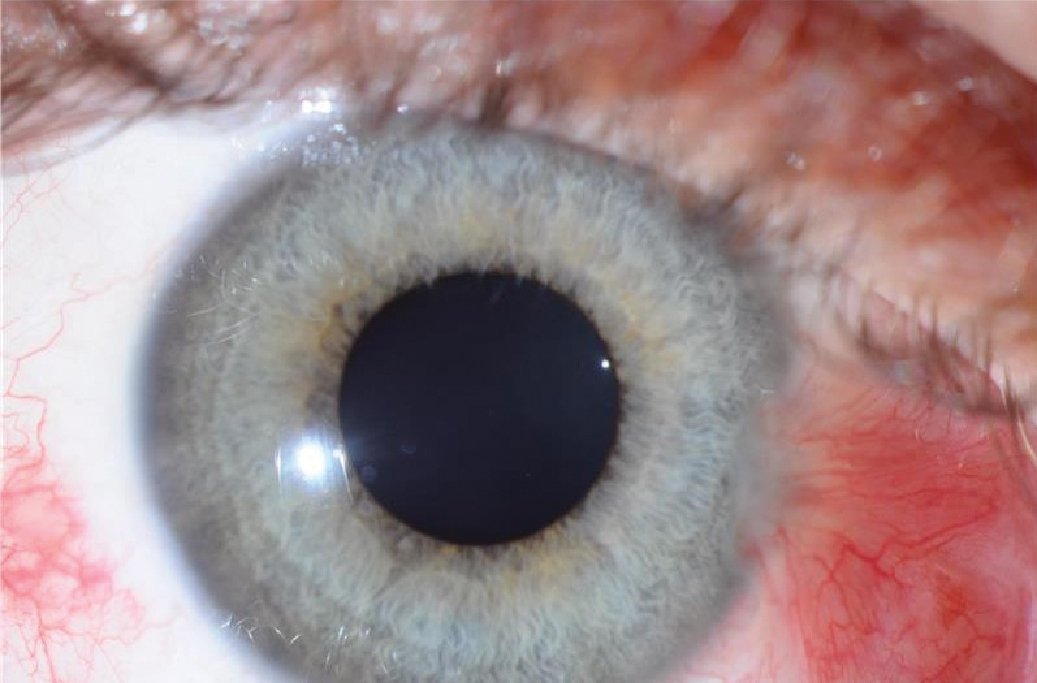
Inflammation is a central component of pterygium pathophysiology. Chronic UV exposure causes oxidative stress on the DNA of conjunctival cells, which triggers an inflammatory cascade in the conjunctiva, characterized by releasing pro-inflammatory cytokines and chemokines.7,8 Inflammatory mediators stimulate the release of growth factors, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), in pterygium tissue.9 Angiogenesis, or the formation of new blood vessels, is promoted by VEGF, and these new blood vessels supply oxygen and nutrients to the developing pterygium and give the characteristic red eye appearance often associated with pterygia (Figure 2).10
 |
|
Fig. 2. Red appearance of eye due to angiogenesis in pterygium tissue. Click image to enlarge. |
Expression of FGF within the conjunctival tissues stimulates the activation of fibroblasts. These normally quiescent cells transform into myofibroblasts, which are highly contractile and capable of synthesizing and depositing extracellular matrix components like collagen and fibronectin.9 Myofibroblasts contribute to the fibrotic nature of pterygium tissue.9
Another consequence of chronic inflammation is the over-expression of matrix metalloproteinases (MMPs), which leads to remodeling the conjunctiva’s extracellular matrix (ECM).11 The cleaving of ECM structural proteins (collagen, fibronectin and proteoglycans) by MMPs achieves the degradation and remodeling of the conjunctival tissue. The presence of MMPs in pterygium tissue contributes to tissue invasion. As MMPs degrade the corneal basement membrane and collagen fibers, they create pathways for pterygium tissue to infiltrate and invade the cornea.8 This is a critical step in the progression of pterygium, as it allows the lesion to encroach further onto the cornea, thereby affecting vision.
While UV radiation remains a prominent risk factor, genetic and molecular factors also contribute to pterygium development. Research has identified specific genetic polymorphisms and molecular alterations associated with an increased susceptibility to pterygium.12 These factors may modulate the individual response to UV radiation and inflammation, further highlighting the relationship between genetics and environmental factors in the condition’s pathophysiology.
Recently, viral infections such as herpes simplex (HSV) and human papilloma (HPV) have been found in pterygium tissue samples.13,14 The role of viruses in the pathophysiology of pterygium progression is controversial, but further research may lead to anti-viral medication options for pterygium treatments.
Diagnostic Assessment
Although pterygium has potentially significant visual and ocular discomfort implications, the accurate diagnosis of the condition is sometimes overlooked. Due to its variable presentation, diagnosis can be difficult, but it is necessary for clinical decision-making. It is helpful for eyecare practitioners to integrate the diagnostic assessment for pterygium into their usual clinical evaluation, especially for those who work in communities with high-risk factors. Living close to the equator, in a hot and dusty environment, older age, males, and many hours of sunlight exposure have all been identified as risk factors for the formation of pterygium.1
 |
| Click table to enlarge. |
A thorough patient history will elucidate exposure to risk factors and determine if the patient has any symptoms associated with pterygium, such as eye irritation, redness, restricted ocular movements or induced astigmatism leading to blurry vision (only in advanced cases where the growth has invaded the cornea significantly).15 Slit-lamp examination remains the cornerstone of pterygium diagnosis as it is an instrument that all eyecare practitioners have access to. It allows for a detailed pterygium size, location and vascularity evaluation.16 Stoker’s line is an iron deposition in the superficial cornea at the head of the pterygium and can also be viewed with a slit lamp.17
Anterior segment optical coherence tomography (AS-OCT) is becoming more popular in assessing and tracking the progression of pterygium as it provides more detail than a slit-lamp evaluation.18 Automated methods using anterior segment images and computer programs are also being developed for screening purposes in rural areas to enhance diagnostic capacity in underserved areas.19 Pterygium can induce astigmatism and an irregular corneal surface, affecting visual acuity (VA).
Measurement of VA, refraction, and topography is necessary to monitor the effect of the pterygium if it has invaded the cornea.
Pterygia can be described as having a head (the part that grows towards the cornea), body (the area that joins the head and base) and a base (the largest part of the pterygium, which lies over the bulbar conjunctiva). The pterygium can be graded once a diagnosis has been established based on the above assessment. Grading of pterygium is commonly based on its clinical characteristics, including size, vascularity and involvement of the cornea. A few grading systems have been proposed in the literature for primary and recurrent pterygia.20-23 Different grading scales are necessary as some recurrent pterygia lack episcleral vessels, which is a feature of some grading systems for primary pterygia.
 |
|
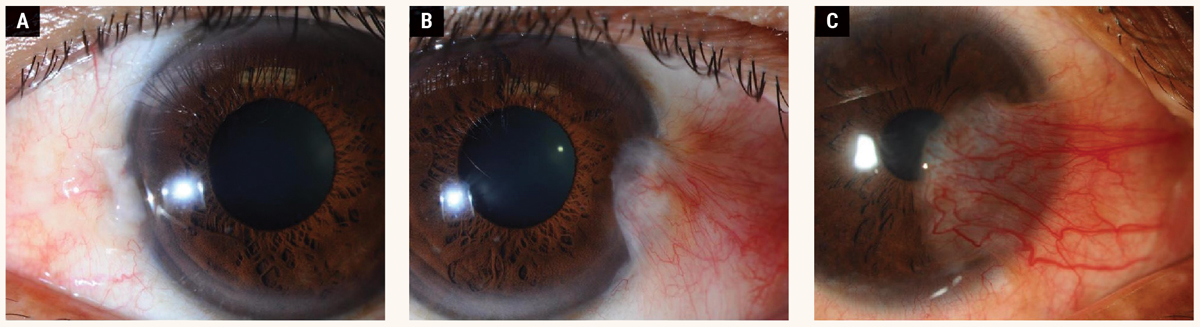
Fig. 3. Grading based on the size of the lesion: (A) Grade 1, (B) Grade 2, (C) Grade 3. Click image to enlarge. |
The Tan classification is a widely used grading system based on morphology (Table 1).20 Table 2 shows the grading system based on size.24 Figure 3 shows the different grades of pterygium based on the scale suggested by one study. 24
Several conditions may mimic pterygium, making differential diagnosis crucial. The differential diagnosis includes nevi, conjunctival lymphoma, limbal dermoid, ocular surface squamous neoplasia (OSSN), pseudo-pterygium and ocular cicatricial pemphigoid.6 Distinguishing between a benign pterygium and a malignant lesion, such as OSSN, requires a comprehensive clinical evaluation and, in some cases, additional diagnostic procedures. There have been cases where OSSN was detected in pterygium samples.25
If there is diagnostic uncertainty or clinical suspicion of malignancy, a biopsy to obtain tissue samples for histopathological examination is necessary. Histopathological examination of tissue samples can provide definitive evidence of malignancy. Dysplastic or malignant epithelial cell features, such as nuclear atypia, increased mitotic activity and abnormal cell architecture, would confirm malignancy.
Management Options
Addressing pterygium involves a range of treatment options depending on the severity of the condition and the presence of associated symptoms. In mild cases where the pterygium is small and asymptomatic, observation with regular follow-up may be appropriate. Patients can use lubricating eye drops to alleviate dryness and discomfort. A steroid eye drop can be prescribed to manage mild ocular irritation and redness symptoms.
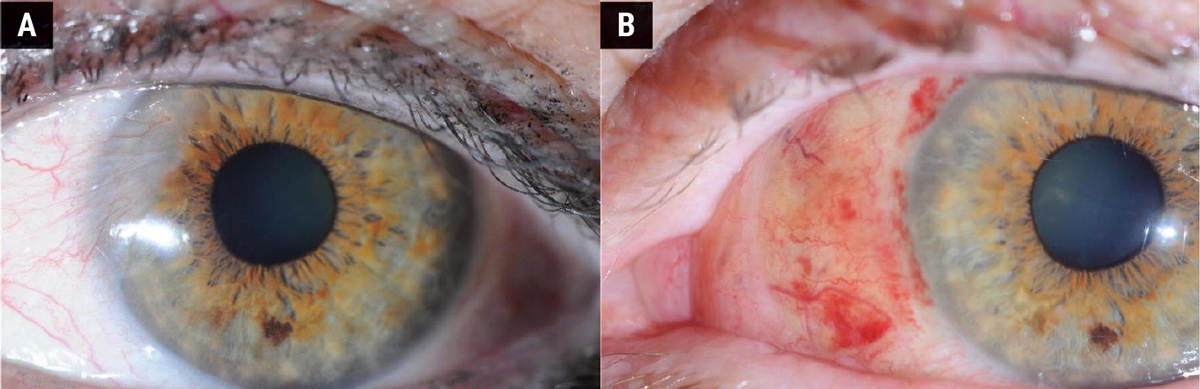
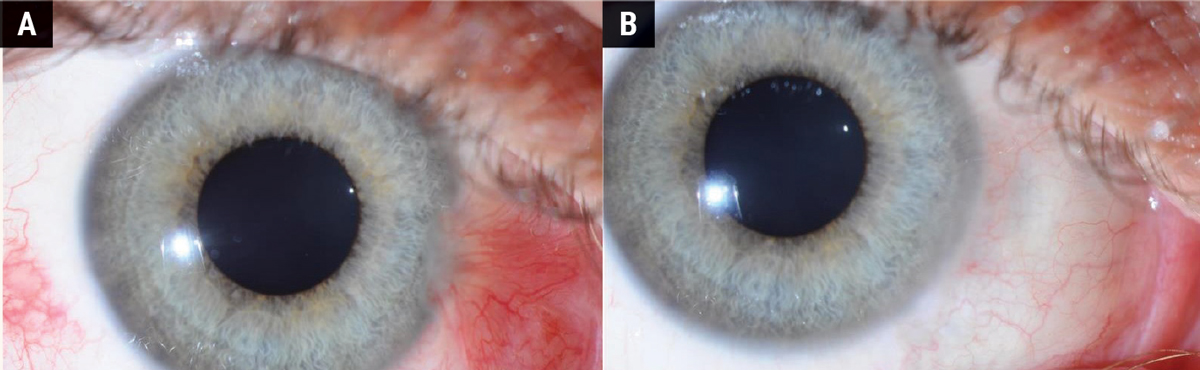
Surgical removal of the pterygium is the primary treatment for cases with significant symptoms, visual obstruction or cosmetic concerns. Figures 4 and 5 show the difference in appearance of the eye before and after surgery. Figure 4 shows a follow-up period of three months where the sclera is still red and recovering from the procedure. Figure 5 shows a fully healed sclera one-year post-surgery.
 |
| Click table to enlarge. |
The chief complication of surgery is the high recurrence rate. Risk factors for pterygium recurrence following surgery are younger age, more advanced stage of disease and untreated postoperative inflammation.26 The recurrence rate has been found to be as high as 97% within the first year postoperative.27
The bare sclera technique is one of the quickest, most straightforward surgical interventions; however, it has a high recurrence rate. An incision is made near the head of the pterygium, and the tissue is removed from the underlying cornea or conjunctiva. Unlike other techniques involving grafting tissue to cover the excision site, the bare sclera technique leaves the sclera exposed after removing the pterygium. The rationale is to minimize the risk of graft-related complications and reduce surgical time.
Depending on the surgeon’s preference and the patient’s condition, adjunctive therapies may be applied to the bare sclera, such as the intraoperative use of mitomycin C (an antineoplastic antibiotic), which aids in reducing the recurrence of pterygium.24 Surgeons have been advised to refrain from using this technique in isolation as it has been shown that pterygia is up to 25-times more likely to recur with this technique.28
 |
|
Fig. 4. (A) Before pterygium removal surgery. (B) Three months after surgery. Click image to enlarge. |
Additional procedures were explored to reduce the recurrence rate after surgical removal. Conjunctival autograft is a more favorable technique as it has a lower rate of recurrence in comparison to the bare sclera technique. Upon pterygium removal, a conjunctival graft is placed over the exposed area and is either sutured or glued on. The conjunctival tissue is grafted from the same eye from the bulbar conjunctiva under the upper lid as it is not exposed to UV radiation and is generally healthy tissue. The site from which the grafted tissue is taken is left exposed as it will re-epithelize.29
A variation of this technique is limbal conjunctival autograft, where limbal stem cells are included in the grafted tissue.30 Amniotic membrane graft is another fairly recent technique in which the graft that covers the excision site is derived from a donor amniotic membrane. Amniotic membrane has proven useful in ophthalmic surgeries as the tissue is similar to corneal tissue in that it has an epithelium, basement membrane and stroma and is capable of re-epithelization and adhesion to the host tissue. In pterygium excision surgery, the membrane is placed with the stromal side on the exposed sclera and attached with fibrin glue or sutures.31 Conjunctival autograft, limbal conjunctival autograft and amniotic membrane graft have all been shown to have lower recurrence rates than the bare sclera technique.32
 |
|
Fig. 5. (A) Before pterygium removal surgery. (B) A fully healed sclera one year after surgery. Click image to enlarge. |
Adjuvant therapy used pre-, post- and/or intraoperatively has been shown to reduce recurrence rates when paired with one of the procedures discussed above. Common adjuvant therapies include mitomycin-C, beta-irradiation, cyclosporine and 5- fluorouracil.33 These adjuvant therapies do, however, have adverse effects, but the latest research suggests that the best outcomes thus far have been achieved with conjunctival autograft and topical cyclosporine.34
Takeaways
Knowledge of this common ocular surface disorder’s pathophysiological mechanisms, diagnostic assessments and the range of management options available remains crucial. Pterygium, primarily driven by chronic UV radiation exposure, inflammation and genetic factors, presents a complex interaction of pathological processes involving epithelial cells, fibroblasts, extracellular matrix remodeling and angiogenesis.
Its diagnostic assessment involves a multifaceted approach, where clinical exam, imaging techniques and histopathological evaluation play vital roles in accurate diagnosis and differentiation from malignant lesions, such as OSSN. Understanding the risk factors, clinical characteristics and histological features is essential for ODs and ophthalmologists to make informed patient care and management decisions.
Regarding management, a spectrum of options is available, ranging from conservative measures for mild cases to surgical interventions for moderate to severe pterygium. Surgical excision remains the primary choice for symptomatic and visually significant pterygium, with techniques like conjunctival autografting and amniotic membrane transplantation to minimize recurrence. Adjunctive therapies, postoperative care and vigilant follow-up are integral to a successful management strategy. For patients and clinicians, the decision on the appropriate approach depends on carefully evaluating clinical factors and patient preferences.
It is also important to emphasize prevention and early intervention in pterygium management. Education on UV protection and eye health is essential to reduce the risk of pterygium development. A multidisciplinary approach involving optometrists, ophthalmologists, pathologists and other eye care specialists is crucial in pursuing optimal patient outcomes. With a deeper understanding of the pathophysiology, accurate diagnostic assessment and a tailored approach to management, we can provide our patients with the best possible care for this challenging ocular condition.
We would like to thank Amy Humphreys from Northern Vision Clinic, Johannesburg for all the images used in this article.
Dr. Chetty is an academic optometrist with a profound passion for specialised contact lenses and keratoconus. She holds a doctoral degree on keratoconus, has presented her research at various international and national conferences and published many peer reviewed articles over the years. She is a fellow of the International Association of Contact Lens Educators (IACLE) and of the British Contact Lens Association. She is also the IACLE Ambassador for Africa. She is a key opinion leader for Alcon.
Ms. van Zyl is the owner and optometrist at the Low Vision Pretoria practice in the Pretoria Eye Institute. She has been on the committee of the Contact Lens Society of South Africa since 2014 and has organised three international Contact Connect conferences. In 2021, she joined Saks, Taylor and Brauer Optometrists, one of the oldest practices in South Africa, where she spends most of her week. She has no financial disclosures.
1. Rezvan F, Khabazkhoob M, Hooshmand E, et al. Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol. 2018;63(5):719-35. 2. Fotouhi A, Hashemi H, Khabazkhoob M, et al. Prevalence and risk factors of pterygium and pinguecula: the Tehran Eye Study. Eye. 2009;23(5):1125-9. 3. Lin SF, Tsai RK, Tung IC, et al. An epidemiologic study of pterygium in middle-aged and elderly aboriginal populations of the Tao Tribe of Orchid Island in Taiwan. Tzu Chi Med J. 2006;18(4):283-6. 4. Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68(5):343-6. 5. Chui J, Di Girolamo N, Wakefield D, et al. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6(1):24-43. 6. Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021;13: 25158414211020152. 7. Kau HC, Tsai CC, Lee CF, et al. Increased oxidative DNA damage, 8-hydroxydeoxy-guanosine, in human pterygium. Eye. 2006;20(7):826-31. 8. Di Girolamo N, Chui J, Coroneo MT, et al. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog in Retin Eye Res. 2004;23(2):195-228. 9. Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-β and tumor necrosis factor-α in the pterygium. Acta Histochemica. 1996;98(2):195-201. 10. Livezeanu C, Craitoiu MM, Manescu R, et al. Angiogenesis in the pathogenesis of pterygium. Rom J Morphol Embryol. 2011;52(3):837-44. 11. Dushku N, John MK, Schultz GS, et al. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119(5):695-706. 12. Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007;18(4):308-13. 13. Chalkia AK, Spandidos DA, Detorakis ET. Viral involvement in the pathogenesis and clinical features of ophthalmic pterygium. Int J Mol Med. 2013;32(3):539-43. 14. Rodrigues FW, Arruda JT, Silva RE, et al. TP53 gene expression, codon 72 polymorphism and human papillomavirus DNA associated with pterygium. Genet Mol Res. 2008;7(4):1251-8. 15. Wanzeler AC, Barbosa IA, Duarte B, et al. Mechanisms and biomarker candidates in pterygium development. Arq Bras Oftalmol. 2019;82:528-36. 16. Mohammad-Salih PA, Sharif AF. Analysis of pterygium size and induced corneal astigmatism. Cornea. 2008;27(4):434-8. 17. Arai Y, Makino S, Obata H. Stocker’s line in pterygium. J Gen Fam Med. 2017;18(2):92-3. 18. Batur M, Seven E, Tekin S, et al. The role of anterior segment optical coherence tomography in the evaluation of the pterygium. Photodiagnosis Photodyn Ther. 2023;43:103704. 19. Zaki WM, Daud MM, Abdani SR, et al. Automated pterygium detection method of anterior segment photographed images. Comput Methods Programs Biomed. 2018;154:71-8. 20. Tan DT, Chee SP, Dear KB, et al. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115(10):1235-40. 21. Maheshwari S. Pterygium-induced corneal refractive changes. Indian J Ophthalmol. 2007;55(5):383. 22. Prabhasawat P, Barton K, Burkett G, et al. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104(6):974-85. 23. Liu J, Fu Y, Xu Y, et al. New grading system to improve the surgical outcome of multirecurrent pterygia. Arch Ophthalmol. 2012;130(1):39-49. 24. Verma NI, Garap JA, Maris RO, et al. Intraoperative use of mitomycin C in the treatment of recurrent pterygium. P N G Med J. 1998;41(1):37-42. 25. Van Acker SI, Van den Bogerd B, Haagdorens M, et al. Pterygium—the good, the bad, and the ugly. Cells. 2021;10(7):1567. 26. Janson BJ, Sikder S. Surgical management of pterygium. Ocul Surf. 2014;12(2):112-9. 27. Hirst LW, Sebban A, Chant D. Pterygium recurrence time. Ophthalmology. 1994;101(4):755-8. 28. Sánchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998;82(6):661-5. 29. Cioba C, Marafon SB, Fortes BG, et al. Autologous fibrin glue versus sutures for conjunctival autograft in primary pterygium: a randomized clinical trial. Int Ophthalmol. 2023;43(7):2371-81. 30. Mery G, Maalouf T, George JL, et al. Limbal-conjunctival autograft in pterygium surgery. J Fr Ophtalmol. 2010;33(2):92-8. 31. Jirsova K, Jones GL. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18:193-204. 32. Paganelli B, Sahyoun M, Gabison E. Conjunctival and limbal conjunctival autograft vs. amniotic membrane graft in primary pterygium surgery: a 30-year comprehensive review. Ophthalmol Ther. 2023;12(3):1501-17. 33. Fonseca EC, Rocha EM, Arruda GV. Comparison among adjuvant treatments for primary pterygium: a network meta-analysis. Br J Ophthalmol. 2018;102(6):748-56. 34. Chu WK, Choi HL, Bhat AK, et al. Pterygium: new insights. Eye. 2020;34(6):1047-50. |
